INTRODUCTION
Asia has the opportunity to be a global leader in combating the growing epidemic of chronic kidney disease (CKD). This opportunity should be based on our knowledge of the strengths (and weaknesses) of the Asian medical and scientific communities and the environments in which they work.
DEMOGRAPHIC ISSUES IN ASIA
Nearly 50% of the global population of nearly 7 billion persons live in the Asian Pacific region (1). Six of the 10 most populous nations in the world are in Asia, including the 2 largest (China 1.3+ billion, India 1.1+ billion) along with Indonesia, Pakistan, Bangladesh and Japan. Population densities range from some of the world's highest (Macau, Singapore, Hong Kong, Maldives, and Bangladesh-all over 1,000 persons per km2) to some of the lowest (Mongolia and Australia-both less than 3 persons per km2).
Asia has both strengths and weaknesses relating to this large population. In terms of annual gross domestic product (GDP) 3 of the world's 4 wealthiest nations are in Asia (US$GDP Japan 4.3 × 109, China 3.3 × 109, India 1.1 × 109), and the GDP per capita is over US$30 × 103 in Singapore, Hong Kong, Brunei, Australia, Japan, and Taiwan; ranking them in the 22 highest incomes per person in the world (2). However we also have some of the poorest of people, with GDP per capita being less than US$2,000 p.a. in Afghanistan, Burma, Nepal, Bangladesh, Cambodia, The Solomons, and Papua. The Solomons and Papua New Guinea, and total economies of GDP less than US$500 million in East Timor, the Solomon and Tonga (2).
Though the large population of Asia brings its own challenges, the world's pharmaceutical industry has recognised its strength, with increasing numbers of prospective therapeutic trials being carried out in Asia. Regrettably however, a recent survey found that of 13,152 commercially sponsored trial sites in the world, Asia had only 2 trial sites per million population, compared with North America 191 and Europe 86 (3). This strongly suggests that Asian populations are not being adequately recruited into clinical trials, and hence properly represented in efficacy and side-effect databases. We must move to accelerate the trend towards using Asian populations in sponsored clinical trials.
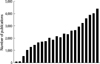
Scientifically this population has contributed remarkably to publications in the last few decades (Fig. 1). In addition to this, a growing number of publications from institutions in western countries have authors born in Asia or descended from Asian parentage. This scientific basis is already enhancing the two-way communication between Asian and the rest of the world and is likely to result in Asian leadership over the next decades.
The cultural diversity within the Asian Pacific region is arguably greater than any other area in the world. Underpinning the fascinating variety of religions and languages are important and unique behaviours and values which, though fundamentally similar, differ in many ways. But in terms of CKD, the influences of everyday behaviour can have enormous impacts on options for patients and their carers, influencing fundamental decisions such as live and deceased donor transplantation, self-care or dependent-care, healthcare funding processes, as well as solutions to clinical problems. The solutions on CKD problems, varying according to these values, should provide a basis for combating CKD world-wide.
SPECIAL ASIAN ISSUES
A particularly intriguing issue in the Asian Pacific region is the striking variance in incidence and prevalence of endstage kidney disease (ESKD) as recognised by rates of dialysis and transplantation. In countries with the economic power to offer dialysis to virtually all citizens, Japan (4) and Taiwan (5) have the world's highest incidence and prevalence of ESKD with those of Korea rapidly accelerating (6), while Australia has amongst the lowest. This enormous disparity has been partially related to the impact of diabetes and ageing in Asia (7) but also seems to involve other factors (8-13).
One study of CKD in our region concluded that the reason for the high rates seen in more wealthy Asian areas was a combination of increasing incidence of type 2 diabetes and ageing with an underlying propensity for CKD to progress more quickly in non-European Asian populations (7). The widespread social and dietary deprivation of the first half of the last century, combined with the rapid improvements over the last 50 yr may be responsible for this, according to the theory relating malnutrition to low birth weight, fewer pancreatic islets and fewer nephrons; hence later life type 2 diabetes and progression of CKD (8). It is not quite that simple. No doubt important ethnic (genetic) influences play a role, as well as environmental factors such as nutrition, infection and exposure to toxins (8-13).
Internationally, but stemming mainly from Western countries, have come classifications and recommended treatment regimens for a wide variety of CKD issues. Inherent in these is the assumption of a relatively restricted variety of population. Perhaps this is most evident when we look at formulae for calculating a glomerular filtration rate (GFR) from plasma creatinine. The first widely applied formula, resulting in an estimate of creatinine clearance (CrCl), was the Cockroft-Gault formula which was developed in a Caucasian Canadian group of 249 patients, using gender, weight and plasma creatinine (14). The next major step forward was the formula from the Modification of Diet in Renal Disease (MDRD) Study, which recognised some ethnicity effects with a correction to be applied if the patient is black (15). Critically, since there was no data on Asian persons in the MDRD study, there was no correction available for the Asians. The lack of specific GFR formulae for Asian populations is not an insignificant issue, since it reflects not only on the diagnosis and management of those with established CKD, but also on any attempts to determine the incidence of CKD in our communities. Fortunately this is rapidly being addressed. GFR formulae applicable in Japan (16) and China (17) have been published, and other countries are rapidly following this lead, either testing the validity of previous formula in their community as in Korea (18), or attempting new constructs. A substantive South-Asian formula is eagerly anticipated.
SPECIAL ASIAN DISEASES
While it is clear that Asia is unfortunately following and perhaps overtaking the Western world in the increase in metabolic syndrome and its related renal consequences, we must not lose sight of other diseases which are more common in Asia than in the West (Table 1). This allows concentration on diseases that are locally preventable, and the knowledge gained may have global relevance. In developing countries, poor water supplies, isolated communities and a hot climate contribute to a high incidence of kidney stones (19) and acute renal failure due to gastroenteritis, kidney stones and peri partum bleeding (20, 21). Because of the preponderance of rural dwellers and heavily forested areas, a wide variety of reptile and insect bites cause acute renal failure (22-24). Tropical infections are commonly complicated by renal disease (25-30), including haemorrhagic fever with renal syndrome, where Korea has played a major role in defining the clinical aspects and relationship to the Hantaviruses (28-30). The rapid transition to industrial societies has had adverse environmental effects, resulting in industrial poisoning, and in rural communities improper use of insecticides contributes to both acute and chronic renal failure (31-34). The ingestion of various fungi and fruit has been associated with renal injury, both acute and chronic (35-37).
In addition, there are areas of Asia where pockets of unusual disease occur; their causes are likely to be environmental or genetic but they are as yet unknown. In Thailand, renal tubular acidosis, hypokalaemic periodic paralysis and renal stone disease occur (38). North Central Sri Lanka has an area where chronic tubulointerstitial nephritis is common, similar to aristolochic acid nephropathy, but without evidence of fungal ingestion. Renal tubular acidosis has been reported in areas of Papua New Guinea, possibly related to ovalocytosis (39, 40). Consequent upon these regional diseases, Asian Pacific nephrology has a major opportunity to contribute to understanding their pathophysiology and management.
Less fortunately, however, most of Asia seems prone not only to increased renal complications from the metabolic syndrome, but also it has been suggested that there are more common or severe examples of other diseases, particularly SLE (41-43) and IgA nephropathy (44, 45) than in most European communities. The challenge of CKD to Asia will certainly involve these more traditionally Western issues.
THE FUTURE
Asian nephrology is in an exciting phase. Many have recognised its potential, and the benefits of a committed and collaborative approach. The Asian Pacific Society of Nephrology (APSN) of which the Korean Society of Nephrology is a Sponsor Society is now 25 yr old and every Asian national nephrology society in our region is affiliated. Its journal, Nephrology, is MEDLINE listed and in its 13th year. The next Congress of the APSN is to be held in Korea in 2010. In 2007 the Asian Forum on Chronic Kidney Disease Initiative (AFCKDI) held the first of what have become annual meetings to discuss the issues of collaborating CKD in our region. The 4th AFCDI will likely be held in Korea with the APCN. Global bodies have quickly become involved with both the APCN and AFCKDI meetings, with ISN COMGAN and the Kidney Disease Improving Global Outcomes (KDIGO) represented at all meetings.
Asian can lead nephrology in the 21st century. It is now up to all of us to contribute to this collaborative effect.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download