Abstract
The aim of this study was to investigate whether green tea extract (GTE) has the protective effects on excess L-arginine induced toxicity in human mesangial cell. Human mesangial cells treated with L-arginine were cultured on Dulbecco's modified eagle medium in the presence and absence of inducible nitric oxide synthase (iNOS) inhibitor and GTE. The cell proliferation was determined by 3 (4,5-dimethylthiazol-2-yl)-2, 5-diphengltetrqzolium bromide, a tetrazole assay. The iNOS mRNA and its protein expression were detected by reverse transcription polymerase chain reaction and Western blot, respectively. The concentration of nitric oxide (NO) was measured by NO enzyme-linced immuno sorbent assay kit. L-arginine significantly inhibited the proliferation of human mesangial cells, and induced the secretion of NO to the media. NO production by L-arginine was significantly suppressed by GTE and iNOS inhibitor (p<0.01). The expression level of iNOS mRNA and its protein that was significantly increased by L-arginine was decreased by iNOS inhibitor but not by GTE. GTE protected the mesangial cells from the NO-mediated cytotoxicity by scavenging the NO rather than by iNOS gene expression. Therefore, we conclude that GTE has some protective effect for renal cells against oxidative injury possibly by polyphenols contained in GTE.
Kidney is the main site of endogenous L-arginine synthesis (1, 2). L-arginine used in the protein synthesis is a material for transport, storage and excretion of nitrous products. Especially, L-arginine is main substance of guanidine products such as creatinine and methylguanidine which are considered as uremic toxins. All of these L-arginine metabolites are involved in renal pathophysiology.
Nitric oxide (NO), as a secondary messenger, is formed in the process of oxidation of L-arginine guanidine nitrogen to L-citrulline in cytoplasm. The role of NO in kidney is of importance not only in the modulation of renal hemodynamics but also in the regulation of renal tubular and glomerular functions (3-5). However, inducible nitric oxide synthase (iNOS) pathways which exist in the glomerular mesangial cells, the monocyte, the macrophage and vascular smooth muscle cells are activated by various cytokines in pathologic conditions. The iNOS, induced by many cytokines such as interleukin-1, tumor necrosis factor and lipopolysaccharide, is a mediator to outside injury or inflammation in immune defense mechanism (6). Excess NO is locally formed and damages the kidney. The NO produced by excess arginine as well as the creatine and methylguanidin is the major factor to kidney injury (7, 8).
More and more attentions have been paid to the protective effects of natural antioxidants against drug-induced toxicities especially when free radical generations are involved. Tea has many advantages over chemicals since it contains several antioxidants including polyphenols of catechin and theaflavin (9). Green tea has received a lot of attention owing to its role as an antioxidant and free radicals scavenger (10, 11). It is well known that green tea has anti-cancer, anti-diabetics and anti-hypertensive actions in the clinical studies (12).
In this study, we investigated the antioxidative effects of green tea extract on human mesangial cells with L-arginine induced toxicity.
GTE was prepared from a hot-water extract of green tea (Boseong, Gwangju, Korea) as described by Maity et al. (13). Ten g of green tea leaves were soaked in 100 mL of boiling distilled water for 5 min and filtered.
Human mesangial cell line SV 40 MES 13 (ATCC catalog number CRL-1927) were cultured in Dulbecco's modified eagle medium (DMEM) containing 10% fetal bovine serum (Gibco, Carlsbad, CA, U.S.A.), guanidine 2 mM/L, benzylpenicillin 100,000 U/L, streptomycin 0.1 g/L, human transferrin 2.5 kg/L, sodium selenita 5 µg/L in 5% CO2 incubator at 37℃.
Mesangial cells were cultured with 1×104 density in 96-well microplates. The mesangial cells were divided into four groups. In group A (control group), mesangial cells were cultured without L-arginine, and group B (L-arginine group), L-arginine was given 100 µM/L on 200 µL media with dilution ratio of 4:1. In group C (iNOS inhibitor group), mesangial cells were cultured with L-arginine 100 µM/L with serial dilution plus iNOS inhibitor (L-NAME, 3, 4, and 5 mM/L). In group D (GTE group), mesangial cells were cultured with L-arginine 100 µM/L with serial dilution plus GTE 0.1g (10, 20, and 30 µL) with 10 mL PBS (NaCl 8.0, KCl 0.2, Na2HPO4·12H2O 3.58, and KH2PO4 0.24 g/L). The mesangial cells were cultured for 48 hr.
Cell viability of mesangial cells was measured by 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The incubation medium was completely aspirated upon treatment with L-arginine 0, 26, 33, 41, 51, 64, 80, and 100 µM/L for 2 days and medium was completely removed. The MTT-formazon was added to the cells, and further incubated for 4hr at 37℃. The cells were washed out, and then lysed with 100 µL of fresh dimethyl sulfoxide (DMSO). The absorbance was calculated at 540 nm (A540) using a microplate autoreader (Bio-TEK Instruments, Winooski, VT, U.S.A.).
Tissue nitrite (NO-2) and nitrate (NO-3) were assessed as an index for NO production. Total NO (nitrite plus nitrate) was measured after conversion of nitrate to nitrite by nitrate reductase. The method for measuring nitrite and nitrate levels was based on the Griess reaction (14) and determined by Hsp60 ELISA kit (StressXpress™, Stressgen Biotechnologies Corp., Victoria, BC, Canada).
Total RNA was isolated from mesangial cells using the TRIzol one step method (Gibco, Bethesda, MD, U.S.A.). One µg of total RNA was heated at 70℃ for 10 min and reversely transcribed using reverse transcriptase 200 U (Promega, Madison, WI, USA). The mixture was then incubated at 37℃ for 60 min, heated at 95℃ for 10 min and stored at -20℃ until use. Selected sequences in 5 µL aliquots of cDNA were amplified by PCR 32 cycles using primers for iNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal standard. Cycles consisted of 45 sec of denaturation at 95℃, 45 sec of annealing at 63℃, 1 min of extension at 72℃, followed by 7 min of elongation at 72℃. Primers were designed with computer assistance according to the gene bank. The sequence of the primers are as follows; iNOS sense primer 5'-AGC ATC ACC CCT GTG TTC CAC CA-3', and iNOS anti-sense 5'-TGG GGC AGT AGT CTC CAT TGC CA-3', (Genemed Synthesis Inc., South San Francisco, CA, U.S.A.); GAPDH sense 5'-CCA TCA CCA TCT TCC AGG AGC GAG-3', and GAPDH antisense 5'-TGC CAG TGA GCT TCC CGT TCA GCT C-3'. The iNOS cDNA was normalized by comparison with GAPDH cDNA.
iNOS protein expression was determined by western blot analysis. For the detection of iNOS protein, cells were harvested and incubated for 20 min on ice in lysis buffer and were centrifuged at 14,000 rpm for 20 min.
The samples were subjected to the 10% polyacrylamide gels and transferred to a nitrocellulose membrane at 20 V overnight. Membranes were incubated with anti-iNOS antibodies (1:1,000, Sigma, St. Louis, MO, U.S.A.) for two hours at room temperature. The membranes were then incubated with a conjugated horseradish peroxidase anti-mouse antibody (R&D system, Minneapolis, MN, U.S.A.) for two hours. They were exposed to a radiography film by LumiGLO chemiluminescent substrate and band density were detected by densitometry (LabWorks 4.0, UVP Inc., Upland, CA, U.S.A.).
L-arginine (26, 33, 41, 51, 64, 80, and 100 µM/L) significantly inhibited human mesangial cells proliferation in a concentration-dependent manner compared with that of control group (Fig. 1).
Treatment with L-NAME (iNOS inhibitor, 3, 4, and 5 mM) significantly inhibited the anti-proliferative effect of L-arginine (51 µM/L) in a concentration-dependent manner (Fig. 2).
To determine whether TGE blocks L-arginine-induced toxicity in mesangial cell, cells were cultured with L-arginine treated with GTE. Treatment with GTE (10, 20, and 30 µL) significantly inhibited the anti-proliferative effect L-arginine in a concentration-dependant manner (Fig. 3).
The expression of iNOS mRNA was significantly higher in the L-arginine group than in the control group and lower in the L-NAME group than in the L-arginine group (p<0.05). GTE had no effect on the expression of iNOS mRNA (Fig. 4).
Total amount of iNOS protein expression was significantly higher in the L-arginine group than in the control group and was lower in the L-NAME group than in the L-arginine group (p<0.05), but was not reduced in the GTE group (Fig. 4).
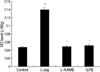
Treatment with L-arginine caused a significant increase in the concentration of NO versus the control group (0.139±0.047 µM/g vs. control 0.047±0.026 µM/g, p<0.01). Increases in NO concentration reduced by L-arginine were significantly suppressed by L-NAME (0.049±0.022 µM/g, p<0.01) and by GTE (0.052±0.032 µM/g, p<0.01, Fig. 5).
Our study revealed that excess L-arginine inhibited the proliferation of mesangial cells by increasing the productions of NO and iNOS. We investigated the effects of GTE on L-arginine-induced cytotoxicity in the mesangial cell, and found that GTE protected the human mesangial cells from L-arginine-induced cytotoxicity by scavenging the NO.
Green tea is produced from the dried leaves of the plant Camellia sinensis and contains several polyphenolic components, such as, (-)-epigallocatechin 3-O-gallate (EGCG), (-)-gallocatechin 3-O-gallate, (-)-epicatechin 3-O-gallate, (-)-epigallocatechin, (+)-gallocatechin, (-)-epicatechin, and (+)-catechin (15). In this study, the components of GTE were EGCG (28% by weight), epigallocatechin (15%), gallocatechin (15%), gallocatechin gallate (10%), epicatechin (7%), epicatechin-3-gallate (5%), catechin (4%). EGCG, the main polyphenol in green tea, has been suggested to participate in the elimination of the uremic toxins, and thus, ameliorate renal disorders (10). The mechanisms of green tea polyphenol were suggested as follows. First, green tea polyphenols increase the bioactivities of superoxide dismutase (SOD) and glutathione peroxidase, which inactivate free oxygen radicals like O2-. Furthermore, because O2- can react with NO by forming ONOO2-, green tea polyphenols may reduce NO loss and maintain NO physical function (16, 17). Second, green tea polyphenol may inhibit the synthesis of thromboxane A2 (TXA2) and leukotrienes (18). The potent vasoconstrictive effects of TXA2 and leukotrienes contribute to the activation of the renin-angiotensin system. There are intrarenal angiotensin II deposits and excessive expression of angiotensin II type I receptor expression has been observed in the renal medulla (19). Future studies are needed to demonstrate the other mechanisms, such as effects of renin-angiotensin systems, of GTE in vitro and vivo.
Excessive dietary arginine evokes renal failure by increasing the production of NO in the kidney. Lui et al. (20) showed that L-arginine could exert in inhibitory effect on the proliferation of human mesangial cells and the production of extracellular components. In the present study, the administration of excess L-arginine inhibited the proliferation of mesangial cells and increased the concentration of NO. Noris et al. (21) reported that arginine levels and NO synthesis were higher in uremic patients than in healthy volunteers, suggesting an explanation for the increased NO synthesis in uremia. Also, in the present study, combined NO2- and NO3- levels was higher in arginine-given mesangial cells than in arginine-free mesangial cells. It raises the possibility that the increase in NO production may be attributable to dietary arginine and that it may cause renal injury.
Therefore, green tea polyphenol suppresses the production of NO and it would be expected to ameliorate the renal injury induced by excessive arginine. Furthermore, green tea polyphenol has a potent scavenging effect through the inhibition of oxidative stress-induced apoptosis in cell culture (22). Yokozawa et al. (23) suggested that excessive arginine affected the activity of antioxidative enzymes in the renal peroxisomes and the reductions in the SOD and catalase activities induced by arginine imply that oxygen-derived free radicals were generated and the biological defense system was weakened but the administration of green tea polyphenol increased the activities of SOD and catalase. In the present study, GTE increased the inhibited proliferation of mesangial cells by L-arginine and suppressed the increase in NO concentration after L-arginine treatment.
In summary, GTE might play a crucial role of NO inhibition as free radical scavenging effect rather than iNOS inhibition. In addition, it was found to ameliorate anti-proliferative effect of L-arginine in mesangial cells, which suggests that GTE, and probably its polyphenols, can protect renal cells against oxidative injury. Future studies are needed to demonstrate the antioxidant effects and other effects of GTE on renal diseases.
Figures and Tables
 | Fig. 1Toxicity of L-arginine on the proliferation of human mesangial cells. Cell viabilities were measured by MTT assay. Indicated amount of L-arginine were added to mesangial cell for 48 hr (n=10 wells. Values expressed as mean±SD. *p<0.05 and †p<0.01 as compared to control group. OD, Optical density. |
 | Fig. 2Effect of iNOS inhibitor (L-NAME) on L-arginine induced human mesangial cells toxicity (96-well microplates). Cell viabilities were measured by MTT assay. Mesangial cells were treated with indicated amounts of L-arginine in the presence and absence of iNOS inhibitor (L-NAME, 3 mM/L, 4 mM/L, and 5 mM/L) for 48 hr (n=9 wells). Values expressed as mean±SD. *p<0.05 as compared to L-arginine group. OD, Optical density. |
 | Fig. 3Effect of green tea extract on proliferation of human mesangial cells (96-well microplates). Cell viabilities were measured by MTT assay. Mesangial cells were treated with indicated amounts of L-arginine in the presence and absence of GTE (10 µL, 20 µL, and 30 µL) for 48 hr (n=9 wells). Values expressed as mean±SD. *p<0.05 and †p<0.01 as compared to L-arginine group. OD, Optical density. |
 | Fig. 4The expression of iNOS mRNA and its protein in human mesangial cells by RT-PCR and Western blot. (A) Mesangial cell pretreated with L-arginine were cultured with iNOS inhibitor (L-NAME) or GTE and were analyzed by RT-PCR for iNOS mRNA. M, molecular marker; C, control; A1, L-arginine 25 µM/L; A2, L-arginine 50 µM/L; A3, L-arginine 100 µM/L; I, iNOS inhibitor 5 mM; G1, GTE 10 µL; G2, GTE 20 µL; G3, GTE 30 µL. (B) Mesangial cell pretreated with L-arginine (50 µM/L) were cultured with iNOS inhibitor (L-NAME, 5 mM) or GTE (30 µL) and were analyzed by Western blot. C, control; A, L-arginine 50 µM/L; A+I, L-arginine 50 µM/L+iNOS inhibitor 5 mM; A+G, L-arginine 50 µM/L+GTE 30 µL. |
References
1. Visek WJ. Arginine needs physiological state and usual diets. A reevaluation. J Nutr. 1986. 116:34–46.

2. Natelson S, Sherwin JE. Proposed mechanism for urea nitrogen reutilization: relationship between urea and proposed guanidine cycles. Clin Chem. 1979. 25:1343–1344.

3. Ito S, Ren Y. Evidence for the role of nitric oxide in the macula densa control of glomerular hemodynamics. J Clin Invest. 1993. 92:1093–1098.
4. Radermacher J, Klanke B, Schurek HJ, Stolte HF, Frolich JC. Importance of NO/EDRF for glomerular and tubular function: studies in the isolated perfused rat kidney. Kidney Int. 1992. 41:1549–1559.

5. Shultz PJ, Schorer AE, Raij L. Effects of endothelium-derived relaxing factor on rat mesangial cells. Am J physiol. 1990. 258:F162–F167.
6. Raij L, Shultz PJ. Endothelium-derived relaxing factor, nitric oxide: Effects on and production of mesangial cell and glomerulus. J Am Soc Nephrol. 1993. 3:1435–1441.
7. Orita Y, Tsubakihara Y, Ando A, Nakata K, Takamitsu Y, Fukuhara Y, Abe H. Effect of arginine or creatinine administration on urinary excretion of methylguanidine. Nephron. 1978. 22:328–336.
8. Paller MS, Hoidal JR, Ferris TF. Oxygen free radicals in ischemic acute renal failure in the rat. J Clin Invest. 1984. 74:1156–1164.

9. Mohamadin AM, El-Beshbishy HA, El-Mahdy MA. Green tea extract attenuates cyclosporine A-induced oxidative stress in rats. Pharmacol Res. 2005. 51:51–57.

10. Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW, Liu W, Bucana CD, Gallick GE, Ellis LM. EGCG, a major component of green tea, inhibits tumor growth by inhibiting VEGF induction in human colon carcinoma cells. Br J Cancer. 2001. 84:844–850.
11. Tsubono Y, Nishino Y, Komatsu S, Hsieh CC, Kanemura S, Tsuji I, Nakatsuka H, Fukao A, Satoh H, Hisamichi S. Green tea and the risk of gastric cancer in Japan. N Engl J Med. 2001. 344:632–636.

12. Ji BT, Chow WH, Hsing AW, McLaughlin JK, Dai Q, Gao YT, Blot WJ, Fraumeni JF Jr. Green tea consumption and the risk of pancreas and colorectal cancer. Int J Cancer. 1997. 70:225–258.
13. Maity S, Vedasiromoni JR, Ganguly DK. Role of glutathione in the antiulcer effect of hot water extract of black tea (Camellia sinensis). Jpn J Pharmacol. 1998. 78:285–292.

14. Cortas NK, Wakid NW. Determination of inorganic nitrate in serum and urine by a kinetic cadmium reduction method. Clin Chem. 1990. 36:1440–1443.
15. Zhang Q, Kelly AP, Wang L, French SW, Tang X, Duong HS, Messadi DV, Le AD. Green tea extract and (-)-epigallocatechin-3-gallate inhibit mast cell-stimulated type I collagen expression in keloid fibroblasts via blocking PI-3K/AkT signaling pathways. J Invest Dermatol. 2006. 126:2607–2613.

16. Morrisey JJ, Ishidoya S, McCracken R, Klahr S. Nitric oxide generation ameliorates the tubulointerstitial fibrosis of obstructive nephropathy. J Am Soc Nephrol. 1996. 7:2202–2212.
17. Shi SH, Zheng SS, Xie HY. Tea polyphenols protect against cyclosporine-induced acute nephrotoxicity in rats. Chin J Organ Transplant. 2001. 22:271–273.
18. Choi JH, Chang HW, Rhee SJ. Effect of green tea catechin on arachidonic acid cascade in chronic cadmium-poisoned rats. Asia Pac J Clin Nutr. 2002. 11:292–297.

19. Giachelli CM, Pichler R, Lombardi D, Denhardt DT, Alpers CE, Schwartz SM, Johnson RJ. Osteopontin expression in angiotensin II-induced tubulointerstitial nephritis. Kidney Int. 1994. 45:515–524.

20. Liu BC, Ma KL, Ye YY, Liu NF, Ruan XZ. Effects of L-arginine on proliferation of human renal mesangial cells and production of extracellular matrix. Acta Pharmacol Sin. 2001. 22:756–760.
21. Noris M, Benigni A, Boccardo P, Aiello S, Gaspari F, Todeschini M, Figliuzzi M, Remuzzi G. Enhanced nitric oxide synthesis in uremia: implication for platelet dysfunction and dialysis hypotension. Kidney Int. 1993. 44:445–450.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download