Abstract
It has been demonstrated that inhibitors of advanced glycation end products (AGE), such as aminoguanidine, can suppress peritoneal AGE in rats on peritoneal dialysis (PD). However, it is unknown whether late administration of a putative cross-link breaker, alagebrium, could reverse peritoneal AGE. We therefore compared alagebrium with aminoguanidine in their ability to reverse peritoneal AGE in rats on PD. Male Sprague-Dawley rats were randomly divided into 3 groups: group I dialyzed with 4.25% glucose solution for all exchanges; group II dialyzed with 4.25% glucose solution containing aminoguanidine, and group III dialyzed with 4.25% glucose solution containing alagebrium for last 8 weeks of 12-week dialysis period. Dialysis exchanges were performed 2 times a day for 12 weeks. Immunohistochemistry was performed using a monoclonal anti-AGE antibody. One-hour PET was performed for comparison of transport characteristics. The immunolabelling of AGE in peritoneal membrane was markedly decreased in the alagebrium group. Consistent with this, the alagebrium group exhibited significantly higher D/Do glucose and lower D/P urea, suggesting low peritoneal membrane transport. But there were no significant differences between the control and the aminoguanidine group. These results suggest that the alagebrium may be the optimal therapeutic approach, compared with treatment with inhibitors of AGE formation, in rats on PD.
Advanced glycation end products (AGE) have been implicated in the pathogenesis of many of the secondary complications of diabetes (1), especially vascular disease. The effects of AGE may also be mediated through interaction with receptors on endothelial, smooth muscle, mesangial, and inflammatory cells, among others (2). Increased serum AGE has been shown to contribute to the accelerated vascular dysfunction associated with uremia and diabetes (3, 4). The formation of AGE has been proposed to result in cross-linking of collagen and distortion of subcelluar structures, causing irreversible tissue damage in peripheral nerves, the macro and microvasculature (5, 6). Prolonged exposure to the glucose-based dialysis fluids, currently in use, results in production and deposition of AGE in the subendothelial area of the peritoneum, inducing microvascular changes similar to those seen in diabetes, and eventually loss of peritoneal function.
Several studies have demonstrated the inhibition of AGE formation. Aminoguanidine inhibits AGE formation and protein cross-linking in vitro (7). Administration of aminoguanidine to rats prevents diabetes-induced AGE formation and cross-linking of arterial wall connective tissue protein in vivo (8); Aminoguanidine also prevents the increased vascular permeability in the aorta, skin, intestine, heart, vena cava, and brain tissue after exogenous injection of AGE in rats (9). However, aminoguanidine cannot cleave or remove preformed tissue AGEs.
More recently, AGE cross-link breakers, such as N-phenacylthiazolium bromide and alagebrium (4, 5-Dimethyl-3-[2-oxo2-phenylethyl]-thiazolium chloride) have been reported to reverse a number of diseases presumably due to cleavage of tissue AGEs (10-12). Alagebrium is a member of a new generation of compounds developed for clinical investigation. It is thought to cleave established AGE cross-links. Alagebrium has been shown to reverse AGE-mediated vascular stiffness in diabetic rats (12) and have improved arterial compliance in aged humans (13). Many studies have reported the effectiveness of alagebrium in a variety of models of diabetic complications (14-16).
Comparison of alagebrium and aminoguanidine, in a long-term peritoneal dialysis animal model, has not been reported. The present study was performed to evaluate, in a rat in vivo model of peritoneal dialysis, the effects of alagebrium and aminoguanidine on reversal of preformed peritoneal AGE and subsequent effects on peritoneal transport characteristics.
The study was performed on 36 male Sprague-Dawley rats weighing between 275 g and 300 g randomly divided into three groups: group I (n=12), control rats were injected intraperitoneally with 4.25% glucose solution for 12 weeks; group II (n=12), rats were intraperitoneally with 4.25% glucose solution containing aminoguanidine (25 mg/kg) for the last 8 weeks of the 12 week; group III (n=12), rats were injected intraperitoneally with 4.25% glucose solution containing alagebrium (2 mg/kg) for the last 8 weeks of the 12 week study.
Prior to completion of the study, 4 animals in group I, 1 animal in group II, and 2 animals in group III were euthanized due to the development of peritonitis, leaving 29 animals for the analysis.
The rats were anesthetized with ether prior to each intraperitoneal injection of dialysis solution. The abdominal wall was shaved and cleaned with an antiseptic agent (Amukin®, Amuchina, Italy). Commercially available dialysis solution containing 4.25% glucose (Perisis®, Boryung Pharma Company, Korea) was injected (25 mL) into the peritoneal cavity using a 20 gauge needle, twice a day at 07:00 and 19:00 hr, 7 days a week for 12 weeks. Prophylactic antibiotics were administered intraperitoneally with the solution; and were changed every 3 days in the following sequence: gentamicin 8 mg/L; ceftazidime 125 mg/L; and vancomycin 25 mg/L, respectively.
All animals were weighed at the time of arrival and at 3, 6, 9, and 12 weeks. The weights were recorded before the morning intraperitoneal injection.
The peritoneal function was assessed at the 12th week by a 1-hr PET using 1.5% glucose solution. A 1.5% glucose solution was used for the overnight exchange preceding the PET. At time 0, 25 mL of dialysis solution was administered intraperitoneally. At 1 hr, the animal was anesthetized, and a sample of dialysate was taken for measurement of urea nitrogen, creatinine, and glucose. Immediately thereafter, a blood sample (2 mL) was taken by direct cardiac puncture for measurement of urea nitrogen, creatinine, and glucose.
The peritoneal membrane transport rate was assessed by dialysate-to-plasma ratio (D/P) of urea nitrogen and D/Do glucose, where D is the glucose concentration in the dialysate after the 1-hr dwell, and Do is the glucose concentration in the dialysis solution before infusion into the peritoneal cavity. High levels of D/P urea nitrogen and low levels of D/Do glucose indicate high transport.
All animals were humanely euthanized during the 12th week, and specimens for immunostaining were obtained. Peritoneal tissues were taken from four locations: a loop of gut with mesentery, abdominal wall, diaphragm, and the surface of liver. Peritoneal sections (4 µm thick) were cut from paraffin blocks.
Immunostaining of AGE in peritoneal tissue sections was conducted using the streptoavidin-biotinylated peroxydase complex method. The sections were washed three times with phosphate buffered saline (PBS). Endogenous peroxidase activity was blocked by incubating the sections with 0.3% H2O2 at room temperature for 30 min. The sections were washed with PBS and incubated with normal goat serum in PBS at room temperature for 2 hr. After the normal goat serum was removed, the sections were incubated in a humid chamber at room temperature overnight with anti-AGE mouse monoclonal antibody (Dojindo Laboratories, Tokyo, Japan) diluted 100 × in PBS. The sections were washed with PBS and incubated with secondary antibody, biotinylated anti-mouse Immunoglobulin G ([IgG] Vector Laboratories, Burlingame, CA, U.S.A.), at room temperature for 1 hr. After washing three times with PBS, the sections were incubated with streptoavidin peroxidase complex (Vector Laboratories) at room temperature for 30 min. After washing three times with PBS, the peroxidase reaction was visualized by incubation with diaminobenzidine for 15 min. After washing with PBS, the slides were counterstained with hematoxylin. Control staining without primary antibody was performed (2 sections in each group) to confirm specificity of the reaction.
The slides were independently read by five separate examiners in a blinded fashion and staining intensity was graded semi-quantitatively from 0 to 3, where 0 represented no staining, 1 represented weak staining, 2 represented moderate staining, and 3 represented strong staining.
Data are presented as mean±standard deviation (SD). Statistical analysis of values at various time points among the groups were assessed by one-way analysis of variance (ANOVA), with Dunn's method for multiple comparisons, using the software SigmaStat 2.0 for Window (SPSS Inc., Chicago, IL, U.S.A.). Differences were considered significant at p<0.05.
Immunostaining showed that AGEs accumulation was weak in the alagebrium group (Fig. 1C, 2C). For the aminoguanidine group, the intensity of staining of the mesothelial layers ranged from moderate to strong (Fig. 1B). Staining of the vascular wall was weakly to moderately positive in this group (Fig. 2B). In the control group, the mesothelial layers had a consistent staining pattern ranging from moderate to strong (Fig. 1A). Staining of the vascular wall was moderately to strongly positive in this group (Fig. 2A).
Fig. 3 shows the scores for AGEs staining by the respective groups. The immunostaining grade was lowest in the alagebrium treated group and highest in the control group. Significant differences were observed in comparisons of the alagebrium group (1.07±0.18) with the aminoguanidine group (2.09±0.45, p<0.05) and with the control group (2.14±0.32, p<0.05).
Table 1 shows D/Do glucose, D/P urea nitrogen and D/P creatinine in the respective groups. Significant differences in D/Do were seen in comparisons of the alagebrium group (0.51±0.03) with the aminoguanidine group (0.44±0.07, p<0.05) and with the control group (0.40±0.07, p<0.05). For D/P urea nitrogen, differences were noted in comparisons of the alagebrium group (0.78±0.05) with the aminoguanidine treated group (0.87±0.10, p<0.05) and with the control group (0.89±0.08, p<0.05). For the D/P creatinine, differences were observed in the comparisons of the alagebrium group (0.78±0.17) with the control group (0.85±0.13, p<0.05).
Table 2 shows changes in weight over time. The mean baseline weight of the rats was approximately 257 g. Weight increased in all groups over the period of study. However, there were no significant differences between groups.
Long-term peritoneal dialysis, using high glucose solutions, results in increased peritoneal AGE accumulation leading to increased peritoneal permeability to small solutes, such as glucose, urea and creatinine. The results of this study show that alagebrium reversed preformed peritoneal AGE accumulation and improved peritoneal permeability. However, treatment with aminoguanidine did not reverse established peritoneal AGE accumulation.
Peritoneal accumulation of AGE has been reported in nondiabetic patients on long-term peritoneal dialysis (17, 18) and has been related to increased peritoneal permeability in long-term PD (17). AGE Cross-linking with the matrix component of capillary basement membrane increases capillary permeability (19, 20). The present findings of increased peritoneal permeability to small solutes, together with strong positive staining for AGE in peritoneal tissues, supports the findings of previous reports.
Aminoguanidine inhibits AGE formation and cross-linking in vivo and in vitro (17). Administration of aminoguanidine to rats inhibits diabetes-induced accumulation of AGEs and abnormal cross-linking of connective tissue proteins in the arterial wall (8). However, aminoguanidine has a limitation that it cannot cleave and remove preformed AGEs from tissue. The results of present study support these observations.
AGE cross-link breakers, such as N-phenacylthiazolium bromide (PTB) and alabebrium (4, 5-Dimethyl-3-[2-oxo2-phenylethyl]-thiazolium chloride) (ALT-711) have been recently reported (10-12). Alagebrium is a member of a new generation of compounds developed for clinical investigation which can presumably cleave established AGE cross-links. These cross-link breakers have been shown to reverse AGE-mediated vascular stiffness in diabetic rats (12) and have improved arterial compliance in aged humans (13). Many studies have reported the effectiveness of alagebrium in a variety of diabetic associated disease models (14-16). In the present study, alagebrium reduced preformed AGEs in capillaries and mesothelial cells in the parietal peritoneum, and improved peritoneal permeability to small solutes, after long-term peritoneal dialysis using high glucose solutions. The present findings of breakdown of preformed peritoneal AGE and prevention of increased peritoneal permeability, observed after long-term peritoneal dialysis using high glucose solutions containing alagebrium, suggest that AGE accumulation in the peritoneum and in the vascular walls resulted in increased capillary permeability to small solutes.
Loss of ultrafiltration is typically associated with increased permeability to small solutes such as creatinine (18). These observations could be explained by AGE accumulation in the peritoneal membrane, resulting in increased permeability to solutes. This might occur via vascular effects or basement membrane thickening, analogous to the situation in diabetes (19). AGE modification of collagen in submesothelial interstitium might diminish the diffusion resistance to small solutes and account for a more pronounced increase in permeability to small solutes than to macromolecules (20). Alternatively, the increased expression of RAGE secondary to AGE accumulation may result in alteration of the matrix components of the vascular walls thus modifying their permeability. In the present study, rats dialyzed with high glucose solution had significantly higher permeability to small solutes compared with rats dialyzed with high glucose solution containing alagebrium. Cross-linking of basement membrane matrix proteins by AGEs such as laminin, heparin sulfate proteoglycan or type IV collagen disturbs the integrity of basement membrane (21, 22), which increases vascular permeability. Expression of RAGE on the vascular endothelium induces an increase in permeability of vascular wall (23). In the present study, AGE staining was evident in the vascular walls of the rats. Accordingly, the accumulation of AGE in the vascular walls of these rats appears to correlate with the increased peritoneal permeability.
Monitoring changes in body weight over time is a useful way of assessing the general condition of experimental rats. Our rats gained weight throughout the study. All rats were dialyzed with high glucose solutions and therefore there were no significant difference in body weights between groups.
The limitation of this study is that there was not a negative control group-rats that do not receive intraperitoneal injection of high glucose solution-in experimental design. So it was hard to compare group I with negative control group. In order to compare the effects of glucose based solutions with those of non-glucose based solutions-specifically, AGE formation in the peritoneal membrane and subsequent effects on peritoneal transport characteristics, a negative control group is necessarily needed. However, this study was to evaluate and compare the ability to cleave preformed peritoneal AGE of aminoguanidine to those of alagebrium with respect to peritoneal transport characteristics and AGE. Thus group I was designed as control group.
In conclusion, the present study demonstrated that intraperitoneal alagebrium could reduce preformed peritoneal AGE and could improve peritoneal permeability in an animal model of long-term peritoneal dialysis. These results suggest that cross link breaker, alagebrium offered a beneficial therapeutic approach when compared with inhibitors of AGE formation, in rats treated with peritoneal dialysis.
Figures and Tables
Fig. 1
Immunostaining for advanced glycation end products in the peritoneal mesothelial cell. The mesothelial cells of group I (A) and group II (B) show moderate to strong expression. However, the mesothelial cells of group III (C) show weak expression.
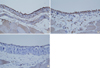
Fig. 2
Immunostaining for advanced glycation end products in the peritoneal vascular wall. The vessels of group I (A) and group II (B) show moderate to strong expression. However, the vessels of group III (C) show weak expression.
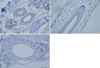
Fig. 3
Semiquantitative scoring of advanced glycation end products immunohistology.
*p<0.05 (control vs. alagebrium); †p<0.05 (aminoguanidine vs. alagebrium).
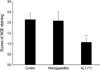
Table 1
Peritoneal transport of glucose, urea nitrogen, and creatinine

Data are presented as mean±standard deviation (SD).
Group I, rats dialyzed with glucose solution; Group II, rats dialyzed with glucose solution containing aminoguanidine; Group III, rats dialyzed with glucose solution containing alagebrium.
*p<0.05 Control group vs. alagebrium group; †p<0.05 Aminoguanidine group vs. alagebrium group.
ACKNOWLEDGMENTS
We are grateful to Boryung pharmaceutical Co. Korea for their kind gift of dialysis solution and to Alteon Inc. U.S.A. for their kind gift of alagebrium.
References
1. Vlassara H, Bucala R, Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, clinical implications for diabetes and aging. Lab Invest. 1994. 70:138–151.
2. Vlassara H. Recent progress on the biologic and clonical significance of advanced glycosylation end-products. J Lab Clin Med. 1994. 124:19–39.
3. Makita Z, Bucala R, Rayfield EJ, Friedman EA, Kaufman AM, Korbet SM, Barth RH, Winston JA, Fuh H, Manogue KR, Cerami A, Vlassara H. Reactive glycosylation end products in diabetic uraemia and treatment of renal failure. Lancet. 1994. 343:1519–1522.
4. Vlassara H. Serum advanced glycosylation end-products: a new class of uremic toxins? Blood Purif. 1994. 12:54–59.
5. Kennedy L, Baynee JW. Non-enzymatic glycosylation and the chronic compliacations of diabetes. An overview Diabetologia. 1984. 26:93–98.
6. Brownlee M, Cerami A, VLassara H. Advanced products of nonenzymatic glycosylation and the pathogenesis of diabetic vascular diseases. Diabetes Metab Rev. 1988. 4:437–451.
7. Lee EA, Oh JH, Lee HA, Kim SI, Park EW, Park KB, Park MS. Structual and fuctional alterations of the poritoneaum after prolonged exposure to dialysis solutions: role of aminoguanidine. Perit Dial Int. 2001. 21:245–253.
8. Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Amionguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986. 232:1629–1632.
9. Vlassara H, Fuh H, Makita Z, Krungkrai S, Cerami A, Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Pro Natl Acad Sci USA. 1992. 89:12043–12047.

10. Cooper ME, Thallas V, Forbes J, Scalbert E, Sastra S, Darby I, Soulis T. The cross-link breaker, N-phenacylthiazolium bromide prevents vascular advanced glycation end-product accumulation. Diabetologia. 2000. 43:660–664.

11. Vasan S, Zhang X, Zhang X, Kapurniotu A, Bernhagen J, Teichberg S, Basgen J, Wagle D, Shih D, Terlecky I, Bucala R, Cerami A, Egan H, Ulrich P. An agent cleaving glucose-derived protein crosslinks in vitro and in vivo. Nature. 1996. 382:275–278.

12. Wolffenbuttel BH, Boulanger CM, Crijns FR, Huijberts MS, Poitevin P, Swennen GN, Vasan S, Egan JJ, Ulrich P, Cerami A, Levy BI. Breakers of advanced glycation end products restore large artery properties in experimental diabetes. Proc Natl Acad Sci USA. 1998. 95:4630–4634.

13. Kass DA, Shapiro EP, Kawaguchi M, Capriotti AR, Scuteri A, DeGroof RC, Lakatta EG. Improved arterial compliance by a novel advanced glycation endproduct crosslink breaker. Circulation. 2001. 104:1464–1470.

14. Forbes JM, Thallas V, Thomas MC, Founds HW, Burns WC, Jerums G, Cooper ME. The breakdown of preexisting advanced glycation end products is associated with reduced renal fibrosis in experimental diabetes. FASEB J. 2003. 17:1762–1764.

15. Forbes JM, Yee LT, Thallas V, Markus Lassila M, Candido R, Jandeleit-Dahm KA, Thomas MC, Burns WC, Deemer EK, Thorpe SR, Cooper MF, Allen TJ. Advanced glycation end product interventions reduce diabetes-accelerated atherosclerosis. Diabetes. 2004. 53:1813–1823.

16. Forbes JM, Cooper ME, Thallas V, Burns WC, Thomas MC, Brammar GC, Lee F, Grant SL, Burrell LA, Jerums G, Osicka TM. Reduction of the accumulation of advanced glycation end products by ACE inhibition in experimental diabetic nephropathy. Diabetes. 2002. 51:3274–3282.

17. Tak WT, Kim SK, Lee JY, Kang HJ, Kim ES, Lee JH. The suppression of peritoneal advanced glycosylation end product formation by intraperitoneal aminoguanidine. Korean J Nephrol. 2006. 25:23–33.
18. Struijk DG, Krediet RT, Kookmen GC, Hoek FJ, Boechoten EW, Reijden HJ, Arisz L. Functional characteristics of the peritoneal membrane in long-term continous ambulatory peritoneal dialysis. Nephron. 1991. 59:213–220.
20. Rippe B. Pathophysiological description of the ultrafiltration changes of the peritoneal membrane during long-term continuous ambulatory peritoneal dialysis. Blood Purif. 1994. 12:211–220.
21. Tsilibary EC, Charonis AS, Reger LA, Dege JE, Furcht LT. The effect of nonenzymatic glycosylation on the binding of the main noncollagenous NC1 domain to the type IV collagen. J Biol Chem. 1998. 263:4302–4308.
22. Chraronis AS, Reger LA, Dege JE, Kouzi-Koliakos K, Furcht LT, Wohlhueter RM, Tsilibary EC. Laminin alterations after in vitro nonenzymatic glycosylation. Diabetes. 1990. 39:807–814.

23. Esposito C, Gerlach H, Brett J, Stern D, Vlassara H. Endothelial receptor-mediated binding of glucose modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989. 170(4):1387–1407.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download