Abstract
We report a patient with progressive supranuclear palsy (PSP) with his serial photographs before the onset of ocular symptoms and after the onset with two year intervals. These photographs show his progressive eyeball deviations toward complete exotropia. There were no effective voluntary eyeball movements, Bell's phenomenon, doll's eye movements, and vestibulo-ocular reflexes. These signs indicate the involvement of the oculomotor nuclear complex by the disease. We suggest that PSP may cause not only 'supranuclear' but also 'nuclear' complete ophthalmoplegia with exodeviation of the eyes.
Progressive supranuclear palsy (PSP) is a neurodegenerative disorder characterized by progressive dystonic axial rigidity, postural instability, pseudobulbar palsy, subcortical dementia, and impaired of voluntary eye movements (1). Facial appearances are characteristic in PSP (1-3). The patients usually show a fixed 'surprised' stare, retracted head, raised eyebrows, markedly decreased blinks (less than 4 per min), and progressive downward gaze palsy. Previously, only a few reports describe PSP patients with photographs (1-4). We report a probable PSP (5) patient with progressive eyeball deviations which was documented with serial photographs.
A 66-yr-old man (Fig. 1A, before the illness) was admitted because of dysphagia with repeated aspirations in March 2007. In October 2003, he was evaluated in a hospital because of blurred vision, dislike of bright lights and easy falls. He remembered several episodes of falling while riding a motorcycle since 2000. These episodes were not associated with feelings of dizziness or faintness and were independent of specific situation or body position. He visited several local eye clinics where he was diagnosed with bilateral cataracts and presbyopia, and underwent bilateral lens implantation in 2000. He did not benefit from the surgery, but felt a progressive worsening toward imbalance and unsteadiness when walking. Eye evaluation, in October 2003, revealed bilateral surgical pseudophakias and moderate exodeviation. Pupils were irregularly shaped and direct light reflexes were sluggish bilaterally. Fundoscopic examinations showed mildly increased cup to disk ratio (0.6 OD and 0.7 OS) with peripapillary atrophy in both eyes. Vertical ocular movements for willed gaze as well as following objects were slow and limited, though full ocular movements were elicited during passive head motions. Volitional horizontal gaze and pursuit of a visual target were also slow with bilateral adduction paresis. However, convergence was normal. Goldmann visual fields were also normal. He was slow and made errors when performing distal rapid alternating movements. He tended to topple backwards. There was neither resting nor action tremor (Fig. 1B). He was diagnosed with 'parkinsonism' and treated with levodopa/benserazide and other dopamine agonists, but his symptoms did not improve. His illness progressively worsened thereafter. He experienced frequent falls and needed a cane to walk. His head was retracted, and his voice was reduced to a slurred growl. He became unable to look down and had difficulties in swallowing (Fig. 1C). In November 2006 and January 2007, he suffered from aspiration pneumonias, and underwent tracheostomy and gastrostomy. Magnetic resonance imaging of the brain demonstrated moderate atrophy of the whole cerebrum and brainstem, as well as mild bihippocampal atrophy (Fig. 1E-G).
He was admitted to our hospital in March 2007. His medical history was otherwise unremarkable. His previous medications included levodopa/carbidopa, ropinirole, selegiline, and afloqualone. His family history was negative for gait, cognitive, or other neurological disorders. He responded correctly to simple verbal commands. His eyes deviated up and lateral, and his vertical and medial eye movements were absent. When the patient attempted to look laterally, only brisk quick lateral eyeball movements were observed on the same side, but the opposite eye showed no movement at all medially. He could not converge his eyes. There was neither Bell's phenomenon nor optokinetic nystagmus. Caloric stimulations with both warm and cold water did not evoke any nystagmus bilaterally. Doll's eye maneuver did not evoke any eyeball movements at all. However, because of the patient's severe nuchal rigidity and retrocollis, the examination was suboptimal. He could not sit in bed by himself, and he repeatedly fell backward without support. His axial rigidity made his distal limb movements appear ataxic but when reaching for close objects with his back supported, his ataxic movements decreased markedly. Reflexes were symmetrical and minimally brisk in the upper and lower extremities with bilateral flexor plantar responses. There was no resting tremor (Fig. 1D).
Pathologically PSP is characterized by the destruction of several subcortical structures, including the substantia nigra, globus pallidus, subthalamic nucleus, and midbrain and pontine reticular formation (6). In addition, recent pathologic criteria for PSP specified that a high density of neurofibrillary tangles and neuropil threads in the basal ganglia and brainstem is crucial for the diagnosis of typical PSP. Neurofibrillary tangles are readily identified in the brainstem including the tegmentum, colliculi, periaqueductal gray, red nucleus, basis pontis, dorsal and median raphe nuclei, and inferior olives, and they also frequently affect the oculomotor nuclear complex and trochlear nucleus (7). Among the earliest signs of PSP is supranuclear gaze palsy. It includes slowing of voluntary downward saccades, a high percentage of errors in the antisaccade task, and frequent presence of square-wave jerks, and progression to a complete vertical gaze palsy (8, 9). The doll's head maneuver may generate a normal vertical vestibular-ocular response that demonstrates the integrity of the third nerve nuclei and confirms that the eye movement disorder is supranuclear. Internuclear ophthalmoplegia (INO), which is an adduction paresis on conjugate horizontal gaze (usually with abduction nystagmus of the contralateral eye), has been described previously in an apparently milder form in a series of 4 patients with PSP (10). The median longitudinal fasciculus (MLF) is thought to represent a final common pathway that enables conjugate horizontal gaze for both volitional and reflex-driven eye movements by connecting the abducens and oculomotor nuclei. Damages to MLF are responsible for INO (11). A previous report described a PSP patient with bilateral INO which were overcome by the vestibule-ocular reflex (3). In our case, we could not elicit any effective eyeball movements in his eyes. From the patient's serial photographs, it is evident that his ophthalmoplegia had been accompanied by progressive eyeball deviation in the upward and outward directions. Lesions in the rostral interstitial nucleus of the medial longitudinal fasciculus (riMLF), interstitial nucleus of Cajal (INC) or posterior commissure may deviate eyeball upward or downward. However, the patient's combined outward eyeball deviation with ophthalmoplegia may indicate a nuclear involvement. The location of the ocular motor nuclei in the brainstem can be an explanation. Contrary to close anatomical relationships between the supranuclear structures and the oculomotor and trochlear nuclei around the midbrain and upper pons, the abducens nucleus is located near pontomedullary junction, thus the pathological processes of the PSP may reach the abducens nucleus later than to the oculomotor and trochlear nuclei from the supranuclear structures or they may never reach that level. We suggest that the earliest ocular findings in PSP may be supranuclear gaze palsy, but as the disease progresses, nuclear level gaze control may also be affected, and exodeviation of the eyeballs may be a clinical manifestation in advanced PSP patients.
Figures and Tables
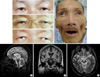
Fig. 1
(A) His axis of binocular vision was normal in his mid-forties. (B) At age 62, his eyes begin to exodeviate. (C) His eyes show more pronounced exodeviation at age 64. (D) His eyes are fully exodeviated at age 66. (E-G) T2-weighted midsagittal (E) and axial (G), and T1-weighted coronal MR images of the patient at age 65. Note the atrophy of the midbrain (E, G), bilateral hippocampus, and mesial frontal lobes (F).
References
1. Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy: a heterogeneous degeneration involving the brainstem, basal ganglia, and cerebellum with vertical gaze and supranuclear palsy, nuchal dystonia and dementia. Arch Neurol. 1964. 10:333–359.
2. Burn DJ, Lees AJ. Progressive supranuclear palsy: where are we now? Lancet Neurol. 2002. 1:359–369.

3. Flint AC, Williams O. Bilateral internuclear ophthalmoplegia in progressive supranuclear palsy with an overriding oculocephalic maneuver. Mov Disord. 2005. 20:1069–1071.

4. Morris HR, Wood NW, Lees AJ. Progressive supranuclear palsy (Steele-Richardson-Olzewski disease). Postgrad Med J. 1999. 75:579–584.
5. Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, Goetz CG, Golbe LI, Grafman J, Growdon JH, Hallett M, Jankovic J, Quinn NP, Tolosa E, Zee DS. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): report of the NINDS-SPSP international workshop. Neurology. 1996. 47:1–9.
6. Jellinger KA, Blancher C. Litvan I, Agid Y, editors. Neuropathology. Progressive supranuclear palsy: clinical and research approaches. 1992. Oxford: Oxford University Press;44–88.
7. Hauw JJ, Daniel SE, Dickson D, Horoupian DS, Jellinger K, Lantos PL, McKee A, Tabaton M, Litvan I. Preliminary NINDS neuropathologic criteria for Steele-Richardson-Olszewski syndrome (progressive supranuclear palsy). Neurology. 1994. 44:2015–2019.

8. Rivaud-Péchoux S, Vidailhet M, Gallouedec G, Litvan I, Gaymard B, Pierrot-Deseilligny C. Longitudinal ocular motor study in corticobasal degeneration and progressive supranuclear palsy. Neurology. 2000. 54:1029–1032.

9. Troost BT, Daroff RB. The ocular motor defects in progressive supranuclear palsy. Ann Neurol. 1977. 2:397–403.

10. Mastaglia FL, Grainger KM. Internuclear ophthalmoplegia in progressive supranuclear palsy. J Neurol Sci. 1975. 25:303–308.

11. Zee DS. Internuclear ophthalmoplegia: pathophysiology and diagnosis. Baillieres Clin Neurol. 1992. 1:455–470.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download