Abstract
We evaluated the efficacy and safety of weekly paclitaxel plus trastuzumab as firs-tline chemotherapy in women with HER2-overexpressing metastatic breast cancer (MBC), and we investigated the prognostic factors including magnitude of HER2/neu amplification in this population. We analyzed 54 patients with HER2-overexpressing MBC that were treated with weekly paclitaxel plus trastuzumab as first-line chemotherapy from February 2004 to December 2006. At a median follow-up of 28 months, median time to progression (TTP) was 16.6 months (95% CI, 9.4 to 23.7 months) and median overall survival was 25.6 months (95% CI, 21.8 to 27.3 months). Therapy was generally well tolerated, although three patients (5.5%) experienced reversible, symptomatic heart failure. Of the 27 patients evaluable for the HER2 FISH, patients with a HER2/CEP17 ratio of ≤4.0 had significantly shorter TTP than those with a HER2/CEP17 ratio of >4.0 (10.8 vs. 23.2 months, P=0.034). A HER2/CEP17 ratio of >4.0 was identified as significant predictive factor of TTP by multivariate analysis (P=0.032). The combination of weekly paclitaxel plus trastuzumab as first-line chemotherapy is an effective regimen in patients with HER2-FISH-positive MBC. Furthermore, the magnitude of HER2 amplification is an independent predictive factor of TTP.
Human epidermal growth factor receptor 2 (HER2) overexpression or amplification has been shown to be associated with a poor prognosis in women with breast cancer (1). Furthermore, trastuzumab, a humanized monoclonal antibody directed against HER2 protein, has been shown to be active against metastatic breast cancer (MBC) overexpressing HER2 (2). In combination with standard chemotherapy, trastuzumab has been found to produce greater response rates and prolong survival in MBC (3). In vitro, HER2 overexpression confers increased resistance to paclitaxel in breast cancer cells, while HER2 degradation increases docetaxel-induced apoptosis (4). In a pivotal randomized phase III trial (5), response rate to paclitaxel was significantly higher in MBC patients when HER2 was downregulated by trastuzumab. HER2 overexpression is also believed to be associated with the efficacy of taxane-based chemotherapy in women with MBC, and trastuzumab may sensitize HER2-overexpressing breast cancer cells to taxanes and result in additive and/or synergistic interactions (6). Common toxicities associated with 3-weekly infusions include neutropenic fever, stomatitis and arthralgia/myalgia, but by administering lower doses of taxanes more frequently, these toxic effects might be reduced while maintaining dose intensity (7). A recent result of a comparative phase III study on weekly paclitaxel and 3-weekly paclitaxel showed the superiority of weekly administration in terms of response rate, time to progression and survival (8). However, by subgroup analyses, trastuzumab did not improve paclitaxel efficacy in HER2 normal MBC. Therefore, weekly paclitaxel regimens represent a valuable therapeutic option for women with MBC, and weekly paclitaxel with trastuzumab in MBC with HER2 overexpression has produced promising results (8-12).
Although trastuzumab is active in patients with HER2-overexpressing MBC, its activity is consistently higher in subsets of patients with an immunohistochemistry (IHC) score of 3+ or gene amplification by fluorescence in-situ hybridization (FISH) than in those with an IHC score of 2+ (5, 8, 12). These findings suggest that response to trastuzumab is substantially dependent on the magnitude of HER2 amplification.
In this study, we evaluated the efficacy and safety of weekly paclitaxel plus trastuzumab in women with HER2-overexpressing MBC. In addition, we investigated whether the magnitude of HER2 amplification is an independent predictor for survival.
We reviewed the records of patients with HER2-overexpressing MBC who had been treated with weekly paclitaxel plus trastuzumab as first-line chemotherapy since 2004 in our hospitals according to the prewritten protocol. Eligibility criteria included: 1) age ≥18 yr with histologically documented metastatic or relapsed HER2 positive breast cancer, 2) no prior chemotherapy in metastatic or relapsed setting, 3) at least one measurable or evaluable lesion, 4) adequate cardiac function evaluated by echocardiography (left ventricular ejection fraction (LVEF) ≥50%) and no prior history of uncontrolled arrhythmia or significant cardiac disease, 5) Eastern Cooperative Oncology Group (ECOG) performance status of 0-2, and 6) adequate hematologic, hepatic and renal function.
Standardized HER2 staining was evaluated by two pathologists in each hospital unaware of clinical information. HER2 IHC results using CB-11 antibody (Novocastra Laboratories, Vision BioSystems, Inc., Norwell, MA, U.S.A.) were scored as 0 when no specific membrane staining was apparent within a tumor and positive when any staining of the tumor cell membranes was observed above the background level. Positive samples were classified semiquantitatively using a 0, 1+, 2+, and 3+ scale, based on their staining intensities. When the staining was heterogeneous, the highest staining intensity was used as the final immunohistochemical result. FISH was performed using PathVysion™ HER2 DNA probe kits (PathVysion; Vysis, Stuttgart-Fasanenhof, Germany) and analyzed as previously described (13). HER2 positivity was defined as an intensity of 3+ by IHC or as gene amplification by FISH. This study was approved by the Institutional Review Board at Seoul National University Hospital.
Paclitaxel plus trastuzumab chemotherapy was administered either at Seoul National University Hospital or at Seoul National University Bundang Hospital. Trastuzumab was administered intravenously (IV) over 90 min at the loading dose of 4 mg/kg on day 1 followed by weekly doses of 2 mg/kg over 30 min. Paclitaxel was administered at 80 mg/m2 IV by 1-hr infusion, following trastuzumab administration every week. Treatment was maintained using this weekly schedule until disease progression or prohibitive toxicity occurred. Paclitaxel treatment was maintained up to 12 cycles at the longest for the patients who were tolerable and did not show progression during treatment, however, paclitaxel was allowed to stop after 6 cycles of treatment when maximal benefit of response obtained according to the investigator's decision and these patients continued to receive single-agent trastuzumab until disease progression. Premedications consisted of dexamethasone 10 mg IV, cimetidine 300 mg IV, and pheniramine 50 mg IV administered 30 to 60 min before paclitaxel infusion. Paclitaxel was administered at full dose if the absolute neutrophil count was >1,500/µL and the platelet count was >100,000/µL. Doses of paclitaxel were reduced in decrements of 10 or 20 mg/m2 if grade 2 or 3 hematologic or nonhematologic toxicities occurred and skipped if grade 4 toxicities occurred. Patients who were responsive to paclitaxel but required discontinuation because of toxicity continued to receive single-agent trastuzumab until disease progression. Trastuzumab was permanently discontinued in patients with symptomatic cardiac events (National Cancer Institute Common Toxicity Criteria [NCI-CTC], grade 3 or 4).
Response assessment was planned every 12 weeks of treatment, according to the Response Evaluation Criteria in Solid Tumor (RECIST) criteria (14). Non-hematologic toxicity and hematologic toxicity evaluations were performed every 2 weeks and were graded according to NCI-CTC version 3.0. Laboratory assessments of blood chemistry and chest radiography were carried out every 4 weeks. LVEF was assessed by echocardiography every 12 weeks and when clinically significant cardiac symptoms developed.
Patients were considered evaluable for the response if they had received at least 12 weeks of treatment or progressive disease at any time. Patients were considered evaluable for toxicity analysis if they had received at least 2 weeks of treatment. Duration of response was defined as the time between first response and disease progression. Time to progression (TTP) was defined as the time from treatment start to disease progression, and was censored at the last date of contact for those that did not experience disease progression. Survival was defined as the time from treatment start until death, and was censored at the date of last contact for those that remained alive. TTP and survival were analyzed using the Kaplan-Meier method and compared using log-rank tests. Multivariate analysis using Cox regression model was performed to investigate the association between clinical outcomes (TTP and survival) and clinical variables, HER2/CEP17 ratio by FISH. The following were regarded as potential explanatory variables; age, hormone receptor status, number of metastatic sites, dominant metastatic site, HER2/CEP 17 ratio by FISH, and subsequent central nervous system (CNS) metastases after treatment initiation. P values of 0.05 or less were considered to indicate statistical significance. All statistical analyses were performed using SPSS for Windows software, version 15.0 (SPSS Inc, Chicago, IL, U.S.A.).
A total of 54 patients with HER2-overexpressing MBC who were treated with weekly paclitaxel plus trastuzumab registered at Seoul National University Hospital (n=42) or Seoul National University Bundang Hospital (n=12) between February 2004 to December 2006. Seven patients had already received taxanes in neoadjuvant or adjuvant setting; the median interval from prior taxane to weekly paclitaxel in the study was 29.5 months (range, 14.8-52.1 months). Baseline characteristics of these patients are summarized in Table 1.
A total of 1,647 weekly infusions of paclitaxel plus trastuzumab and subsequently 613 weekly infusions of maintenance trastuzumab single agent in 14 patients were administered. The median number of weeks of paclitaxel plus trastuzumab was 24.8 (range, 2-64) and subsequent trastuzumab was 35.8 (range, 10-104), and the median delivered dose-intensity for paclitaxel was 70 mg/m2/week, which equated to a median relative dose-intensity of 87.5%. Fifty one (94.4%) of the 54 enrolled patients received at least 12 weeks of treatment. Two patients died of rapid disease progression before 8th week of treatment and one patient was lost to follow up before response assessment. Fifty-one (94.4%) of the 54 enrolled patients were assessable for the response; two patients with only non-measurable bone metastasis were not assessable for the response and one patient was lost to follow up before response assessment, two patients died of rapid disease progression were evaluated as progressive disease. Of the 51 assessable patients, best objective responses included complete response in 9 (17.7%) and partial response in 32 (62.7%); an overall response rate (ORR) of 80.4% (95% CI, 70.9 to 92.5%). Stable disease was observed in 6 assessable patients (11.8%), and only 4 patients (7.8%) experienced disease progression without any period of at least stable disease while on the protocol therapy (Table 2). In this analysis, a total of 30 patients progressed on treatment; the brain was the major site of progressive disease and occurred in 13 patients (25.5% of assessable patients). At a median follow-up of 28 months, median TTP was 16.6 months (95% CI, 9.4 to 23.7 months) (Fig. 1A) and median overall survival was 25.6 months (95% CI, 21.8 to 27.3 months) (Fig. 1B).
All 54 patients who received at least 2 weeks of therapy were assessable for toxicity. In general, the treatment was well tolerated, and the majority of adverse events were of mild to moderate severity (Table 3). Serious hematologic toxicities were infrequent, with grade 3 leukopenia in 11% and grade 3 neutropenia in 22% of patients. However, there were 35 patients (64.8%) who needed dose reduction due to grade 2 or 3 neutropenia. No neutropenic fever occurred. The most common non-hematologic toxicities were peripheral neuropathy and onycholysis. Non-neutropenic infections were observed in 3 patients (6%). One patient suffered from pneumonia after 13 months of treatment and chemotherapy was delayed while antibiotics were administered. Another patient had pulmonary tuberculosis but continued on paclitaxel plus trastuzumab therapy with anti-tuberculosis medication. The third patient withdrew from treatment because of a grade 3 chest wall infection. Grade 3 elevations in transaminases were believed to be associated with underlying fatty liver disease. Paclitaxel was discontinued in seven patients, because of peripheral neuropathy in 5, onycholysis in 1, and a chest wall infection in 1.
The major adverse event associated with trastuzumab was cardiac dysfunction in patients previously exposed to anthracyclines. Seventeen patients developed grade 1 LV systolic dysfunction, and one patient developed grade 2 LV systolic dysfunction. For 18 patients who showed grade 1 or 2 LV systolic dysfunction, the cardiac function has been improved after discontinuation of trastuzumab and the trastuzumab treatment resumed after median 6 weeks. Symptomatic heart failure occurred in 3 patients (6%) who had received anthracycline-based adjuvant chemotherapy. One patient who had been administered a lower cumulative dose of doxorubicin (180 mg/m2) than the others had received adjuvant radiotherapy to the left breast. All 3 patients improved on standard treatments for congestive heart failure.
In the 27 patients with FISH data available, HER2/CEP17 ratios varied from 0.95 to 16.2. Patients with a lower HER2/CEP17 ratio (less than 4.0) had a significantly shorter TTP than those with a higher ratio (10.8 vs. 23.2 months; P=0.034) (Fig. 2, 3). Although a search for predictive factors of survival failed to yield a significant result for any of the investigated variables, multivariate analysis for factors that predict TTP resulted in statistical significance for a HER2/CEP17 ratio of >4.0 (P=0.032) (Table 4).
In this study, we confirmed the efficacy and safety of weekly paclitaxel plus trastuzumab in women with HER2-overexpressing MBC. We obtained an ORR of 80.4% and only 4 (7.8%) of the patients experienced progressive disease as best clinical response. Moreover, the majority of adverse events were mild to moderate in severity and all proved reversible with supportive care. Although it is lower than previous studies (8, 12), the dose intensity of weekly paclitaxel was 87.5%, demonstrating the tolerability of this regimen.
Optimizing the dosing schedule to maximize therapeutic efficacy, while maintaining a favorable toxicity profile, remains an important treatment goal in MBC, and weekly dosing of paclitaxel at a lower dose rather than adopting the standard 3-weekly regimen might better achieve this goal. Several studies have supported weekly paclitaxel as an active regimen in MBC, even when patients were heavily pretreated, had refractory disease, were elderly, or had a poor performance status (15, 16). Furthermore, the encouraging results obtained during single agent trials on weekly paclitaxel in MBC provide a basis for assessing these schedules in combination regimens (17, 18). In these previous studies, hematologic and nonhematologic toxicities were infrequent and dependent on the dose and weekly regimen selected. Based on the above-mentioned studies, weekly paclitaxel and trastuzumab was evaluated in several phase II trials and recent phase III trial involving MBC patients with HER2-overexpressing tumors (8, 10, 12). The Hellenic Cooperative Oncology Group evaluated weekly paclitaxel plus trastuzumab as first line therapy in 34 patients with HER2-overexpressing MBC, and achieved an ORR of 62% and a median TTP of 9 months (10). In addition, a recently reported randomized phase II trial also evaluated the activity of weekly paclitaxel vs. paclitaxel plus trastuzumab for the treatment of patients with HER2-overexpressing MBC, and achieved an ORR of 75% and a median TTP of 10 months (12). One of the interesting findings obtained during this study was that weekly paclitaxel plus trastuzumab was found to be significantly more active in those with an IHC score of 3+ than in those with an IHC score of 2+ (ORR 84.5% vs. 47.5%; P<0.001 and TTP 369 days vs. 272 days; P=0.030), which agrees with our data (ORR 80.4% and TTP 16.6 months).
There are four substantial differences between previous studies on weekly paclitaxel plus trastuzumab and the present study. First, in the present study, HER2 positivity was strictly defined as 3+ by IHC or as amplification by FISH, whereas other studies determined HER2 overexpression based on IHC criteria alone and include the IHC score of 2+ patients. Thus, the higher level of HER2 overexpression adopted in the present study might explain the better responses and longer TTP obtained. Second, we included more hormone receptor (HR) negative patients who are known to have a poorer prognosis than HR positive patients, and although no significant difference was observed, HR negative patients tended to have shorter TTPs and overall survivals than HR positive patients in the present study. Third, the incidence of symptomatic heart failure was higher in the present study, although these cardiac dysfunctions were all reversed by standard cardiac medications. Because of longer TTP of this study, longer treatment duration of trastuzumab might be one of the possible reasons for explanation. Cardiotoxicity is one of the most serious toxicities associated with trastuzumab, as previous major trials have shown (19). Thus, the cardiac functions of patients administered trastuzumab should be carefully monitored, especially those with history of anthracycline use or radiotherapy to the left breast. In the present study, we monitored cardiotoxicity by regular echocardiography, and found seventeen patients with grade 1 LV systolic dysfunction and one patient with grade 2 LV systolic dysfunction. The cardiac function was improved after discontinuance of trastuzumab and the trastuzumab treatment could be resumed after median 6 weeks according to our institutional guideline in all asymptomatic patients if the LVEF is ≥50%. Lastly, there are several differences in non-cardiac toxicities. Most of the patients experienced neutropenia without fever. Grade 1, 2, and 3 neutropenia occurred in 22%, 43%, and 22% of patients, respectively. These results are higher than previous Western studies using even higher dose of paclitaxel 90 mg/m2 which showed 18-29% of grade 1/2 neutropenia and 6-12.7% of grade 3/4 neutropenia (9, 10). In our study, grade 1, 2, and 3 peripheral sensory neuropathy occurred in 15%, 7%, and 6%, respectively. The occurrence of peripheral sensory neuropathy was lower than previous studies using weekly paclitaxel 90 mg/m2 plus trastuzumab for median 25 weeks (range 11-78 weeks) in metastatic breast cancer. There are several reasons to explain the lower incidence of neuropathy. Our study used planned dose of 80 mg/m2 weekly paclitaxel, which was less than 90 mg/m2 in previous studies, and we followed the dose reduction schedule described in the method section if the patients experienced grade 2 or 3 hematologic or non-hematologic toxicities. There were 35 patients (65%) who needed dose reduction due to grade 2/3 neutropenia or grade 2 peripheral sensory neuropathy. Consequently, the median delivered dose intensity of paclitaxel was 70 mg/m2/week, which was lower than that of other studies and this is probably the main reason for explaining less peripheral neuropathy in this study. The median duration of treatment for paclitaxel was similar with other studies. Although it is impossible to compare the median cumulative dose of paclitaxel with other studies, the median cumulative dose of paclitaxel might be lower than previous studies if we extrapolated our data using duration of treatment and dose intensity. In addition, the possibility of underestimation due to a limitation of outcome research is not excluded. Moreover, Korean patients have a tendency to answer vaguely to the neuropathy directed questions.
Brain metastasis (BM) was the major site of progression in the present study. HR negativity and HER2/neu expression are considered to be independent risk factors of BM (20). Patients receiving trastuzumab as first-line therapy frequently develop BM while responding to or with stable disease at other disease sites. It has also been reported that trastuzumab is highly effective at treating liver and lung metastasis in HER2-overexpressing patients, though it is apparently ineffective at treating or preventing BM (21), which is probably explained by its inability to cross the blood brain barrier (22). Since one third of HER2-overexpressing patients with MBC developed BM despite effective trastuzumab treatment, new treatment strategies based on lapatinib or CI-1033 and closer surveillance may be warranted for these patients.
Several studies have shown that FISH is a better determinant of trastuzumab eligibility and a better predictor of prognosis than IHC (23). Furthermore, one retrospective study found that the magnitude of HER2 amplification might be associated with a higher probability of achieving an objective response (24). However, no significant FISH cut-off value was identified in the previous study. We used the PathVysion assay, which included a chromosome-17 probe in a dual-color format. Tumors with a ratio of ≥2.0 HER2 gene copies vs. CEP17 are considered to show HER2 amplification (25). In the present study, when patients were stratified about a HER2/CEP17 ratio cut-off of 4.0, a statistically significant difference in TTP was found between the two groups. In addition, a HER2/CEP17 ratio of >4.0 was found to significantly predict TTP by multivariate analysis. Accurate assessment of HER2 status is essential for determining whether patients are candidates for trastuzumab therapy. Although the present study was based on relatively small number of patients, particularly for HER2 amplification analysis, we suggest that the role of quantitative HER2 gene amplification for predicting efficacy to paclitaxel plus trastuzumab should be considered in order to define an optimal FISH scoring system for the selection of patients that might benefit from trastuzumab-based treatment.
In conclusion, weekly paclitaxel plus trastuzumab is an active regimen in HER2-overexpressing (defined as 3+ by IHC or as amplification by FISH) MBC patients. The present study also suggests that not only HER2 amplification status but also magnitude of HER2 amplification may be a predictive factor for the efficacy of trastuzumab.
Figures and Tables
Fig. 2
Time to progression (TTP) of patients with a HER2/CEP17 ratio ≤4.0 (dotted line) or >4.0 (continuous line). A statistically significant difference was observed (10.8 and 23.2 months, respectively; P=0.034).

Fig. 3
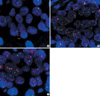
The FISH patterns of HER2/neu gene. The FISH test "highlight" the HER2 genes inside the cell, making them appear as fluorescent signals (dots) so they may be accurately counted. Path-Vysion® FISH also measures the number of copies of chromosome 17 in the cell. Since the HER2 gene resides on chromosome 17, this adds several measures of control to the test. After counting the HER2 and Chromosome 17 signals in 20 nuclei, the ratio of HER2 to Chromosome 17 is calculated. (A) Nuclei with low HER2/neu gene amplification (a HER2/CEP17 ratio of <2.0). (B) Nuclei with intermediate HER2/neu gene amplification (a HER2/CEP17 ratio of 2.0-4.0). (C) Nuclei with high HER2/neu gene amplification (a HER2/CEP17 ratio of >4.0).
References
1. Andrulis IL, Bull SB, Blackstein ME, Sutherland D, Mak C, Sidlofsky S, Pritzker KP, Hartwick RW, Hanna W, Lickley L, Wilkinson R, Qizilbash A, Ambus U, Lipa M, Weizel H, Katz A, Baida M, Mariz S, Stoik G, Dacamara P, Strongitharm D, Geddie W, McCready D. Toronto Breast Cancer Study Group. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. J Clin Oncol. 1998. 16:1340–1349.
2. Valabrega G, Montemurro F, Aglietta M. Trastuzumab: mechanism of action, resistance and future perspectives in HER2-overexpressing breast cancer. Ann Oncol. 2007. 18:977–984.

3. Emens LA, Davidson NE. Trastuzumab in breast cancer. Oncology (Williston Park). 2004. 18:1117–1128.
4. Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004. 96:739–749.

5. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001. 344:783–792.

6. Azambuja E, Durbecq V, Rosa DD, Colozza M, Larsimont D, Piccart-Gebhart M, Cardoso F. HER-2 overexpression/amplification and its interaction with taxane-based therapy in breast cancer. Ann Oncol. 2008. 19:223–232.

7. Eniu A, Palmieri FM, Perez EA. Weekly administration of docetaxel and paclitaxel in metastatic or advanced breast cancer. Oncologist. 2005. 10:665–685.

8. Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, Gipson G, Burstein H, Lake D, Shapiro CL, Ungaro P, Norton L, Winer E, Hudis C. Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol. 2008. 26:1642–1649.

9. Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, Panageas KS, Arroyo C, Valero V, Currie V, Gilewski T, Theodoulou M, Moynahan ME, Moasser M, Sklarin N, Dickler M, D'Andrea G, Cristofanilli M, Rivera E, Hortobagyi GN, Norton L, Hudis CA. Weekly trastuzumab and paclitaxel therapy for metastatic breast cancer with analysis of efficacy by HER2 immunophenotype and gene amplification. J Clin Oncol. 2001. 19:2587–2595.

10. Fountzilas G, Tsavdaridis D, Kalogera-Fountzila A, Christodoulou CH, Timotheadou E, Kalofonos CH, Kosmidis P, Adamou A, Papakostas P, Gogas H, Stathopoulos G, Razis E, Bafaloukos D, Skarlos D. Weekly paclitaxel as first-line chemotherapy and trastuzumab in patients with advanced breast cancer. A Hellenic Cooperative Oncology Group phase II study. Ann Oncol. 2001. 12:1545–1551.
11. Gori S, Colozza M, Mosconi AM, Franceschi E, Basurto C, Cherubini R, Sidoni A, Rulli A, Bisacci C, De Angelis V, Crinò L, Tonato M. Phase II study of weekly paclitaxel and trastuzumab in anthracycline-and taxane-pretreated patients with HER2-overexpressing metastatic breast cancer. Br J Cancer. 2004. 90:36–40.
12. Gasparini G, Gion M, Mariani L, Papaldo P, Crivellari D, Filippelli G, Morabito A, Silingardi V, Torino F, Spada A, Zancan M, De Sio L, Caputo A, Cognetti F, Lambiase A, Amadori D. Randomized Phase II Trial of weekly paclitaxel alone versus trastuzumab plus weekly paclitaxel as first-line therapy of patients with Her-2 positive advanced breast cancer. Breast Cancer Res Treat. 2007. 101:355–365.

13. Lebeau A, Deimling D, Kaltz C, Sendelhofert A, Iff A, Luthardt B, Untch M, Löhrs U. Her-2/neu analysis in archival tissue samples of human breast cancer: comparison of immunohistochemistry and fluorescence in situ hybridization. J Clin Oncol. 2001. 19:354–363.

14. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. European Organization for Research and Treatment of Cancer. National Cancer Institute of the United States. National Cancer Institute of Canada. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000. 92:205–216.

15. Seidman AD, Hudis CA, Albanell J, Tong W, Tepler I, Currie V, Moynahan ME, Theodoulou M, Gollub M, Baselga J, Norton L. Dose-dense therapy with weekly 1-hour paclitaxel infusions in the treatment of metastatic breast cancer. J Clin Oncol. 1998. 16:3353–3361.

16. Perez EA, Vogel CL, Irwin DH, Kirshner JJ, Patel R. Multicenter phase II trial of weekly paclitaxel in women with metastatic breast cancer. J Clin Oncol. 2001. 19:4216–4223.

17. Frasci G, D'Aiuto G, Comella P, Thomas R, Botti G, Di Bonito M, De Rosa V, Iodice G, Rubulotta MR, Comella G. Southern Italy Cooperative Oncology Group (SICOG). Weekly cisplatin, epirubicin, and paclitaxel with granulocyte colony-stimulating factor support vs triweekly epirubicin and paclitaxel in locally advanced breast cancer: final analysis of a sicog phase III study. Br J Cancer. 2006. 95:1005–1012.

18. Loesch D, Robert N, Asmar L, Gregurich MA, O'Rourke M, Dakhil S, Cox E. Phase II multicenter trial of a weekly paclitaxel and carboplatin regimen in patients with advanced breast cancer. J Clin Oncol. 2002. 20:3857–3864.

19. Tan-Chiu E, Yothers G, Romond E, Geyer CE Jr, Ewer M, Keefe D, Shannon RP, Swain SM, Brown A, Fehrenbacher L, Vogel VG, Seay TE, Rastogi P, Mamounas EP, Wolmark N, Bryant J. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005. 23:7811–7819.

20. Gabos Z, Sinha R, Hanson J, Chauhan N, Hugh J, Mackey JR, Abdulkarim B. Prognostic significance of human epidermal growth factor receptor positivity for the development of brain metastasis after newly diagnosed breast cancer. J Clin Oncol. 2006. 24:5658–5663.

21. Stemmler HJ, Kahlert S, Siekiera W, Untch M, Heinrich B, Heinemann V. Characteristics of patients with brain metastases receiving trastuzumab for HER2 overexpressing metastatic breast cancer. Breast. 2006. 15:219–225.

22. Stemmler HJ, Schmitt M, Willems A, Bernhard H, Harbeck N, Heinemann V. Ratio of trastuzumab levels in serum and cerebrospinal fluid is altered in HER2-positive breast cancer patients with brain metastases and impairment of blood-brain barrier. Anticancer Drugs. 2007. 18:23–28.

23. Pauletti G, Dandekar S, Rong H, Ramos L, Peng H, Seshadri R, Slamon DJ. Assessment of methods for tissue-based detection of the HER2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000. 18:3651–3664.

24. Giuliani R, Durbecq V, Di Leo A, Paesmans M, Larsimont D, Leroy JY, Borms M, Vindevoghel A, Jerusalem G, D'Hondt V, Dirix L, Canon JL, Richard V, Cocquyt V, Majois F, Reginster M, Demol J, Kains JP, Delree P, Keppens C, Sotiriou C, Piccart MJ, Cardoso F. Phosphorylated HER-2 tyrosine kinase and Her-2/neu gene amplification as predictive factors of response to trastuzumab in patients with HER-2 overexpressing metastatic breast cancer (MBC). Eur J Cancer. 2007. 43:725–735.





 PDF
PDF ePub
ePub Citation
Citation Print
Print







 XML Download
XML Download