Abstract
Soy-isoflavones may act as estrogenic agonists or antagonists depending on the endogenous hormone status. These clinical effects can be exerted variably in individuals by the metabolic ability to produce a more potent metabolite than precursors. The objective of this randomized, double-blind, placebo-controlled study was to investigate the skeletal effect of isoflavones according to their metabolic variability in premenopausal women. Volunteers were randomly assigned to receive either soy-extract isoflavones (n=32) or lactose (n=21) once a day for three menstrual cycles. After intervention, the urinary excretions of isoflavones and their metabolites were significantly higher in the soy group than in the placebo group and showed a large inter-individual variation. Women in the soy group were divided into subgroups according to their ability to excrete more potent metabolites. Serum osteocalcin and urine deoxypyridinoline showed a tendency to increase after a challenge in equol high-excretors. Serum osteocalcin concentration in the genistein high-excretors increased significantly after a challenge (P=0.04) but did not increase in either the placebo or genistein low-excretors. An estrogenic antagonistic effect of isoflavones on bone turnover was observed in premenopausal women who are able to produce more potent metabolites.
Phytoestrogens are non-steroidal plant compounds and consist of numerous classes including isoflavones, lignans, and coumestans (1, 2). Structurally and functionally, they mimic mammalian estrogens and possess both estrogenic and antiestrogenic properties. The mechanism of their dual functions has not been completely clarified yet. Some in vitro studies have suggested that the biological action of phytoestrogens may be modulated by the concentration of co-existing estrogens; they can act estrogenically in a low-estrogen environment or antiestrogenically in a high-estrogen environment (3, 4). However, only limited data have been available from human studies. Intervention studies with the supplementation of phytoestrogens have revealed inconsistent results on the clinical effectiveness of cardiovascular, menopausal, and bone health.
The high intake of soy isoflavones has been shown to prevent bone loss in some studies of postmenopausal women (5-8). However, these data are still controversial, and they have shown no significant effects on bone in other studies (9, 10). Only a few studies have focused on the skeletal effect of phytoestrogens in premenopausal women. The three-year observational study revealed that soy intake had a significant effect on the maintenance of peak bone mass in women between the ages of 30 and 40 (11), while the other intervention studies showed no effects on bone mineral density (BMD) in young women (12, 13).
Isoflavones are the best-studied phytoestrogens and are found in their highest amounts in soy foods. Recent studies have demonstrated that the metabolism of isoflavones is highly variable in individuals, which suggests that a new paradigm considering this heterogeneity would be indispensable toward an evaluation of their clinical effects (14). Genistin and daidzin, which exist as inactive glucosides, are the two major ingredients of soy isoflavones. Once ingested, they are converted to genistein (GTN) and daidzein (DZ), respectively after breakdown by intestinal microflora. GTN is partially metabolized to its non-estrogenic metabolite, para-ethylphenol, by gut microflora. Some DZ is also further metabolized to equol (EQL) or O-desmethangiolensin (O-DMA) by different metabolic pathways (15-17) (Fig. 1). Previous in vitro studies have shown that the metabolites of the O-DMA pathway have weaker estrogenic properties, while EQL is superior to all other daidzin metabolites, in respect to its binding affinities to estrogen receptors (ERs) and estrogen activities (4). Because of its slower clearance, the plasma concentration of EQL is normally far in excess of other daidzin metabolites (14). However, EQL is not produced in all healthy adults in response to a dietary challenge with soy or DZ; only one-third of healthy adults are capable of producing EQL from DZ. The inter-individual variance in the ability to produce more potent metabolites, including EQL, may lead to differences in the effects of isoflavone intervention on human health. The failure to distinguish these subtypes could plausibly explain the variance in the reported data on the skeletal effects of soy.
This study tested the hypothesis that soy isoflavones may compete with endogenous estrogens and thus act as estrogen antagonists, especially in premenopausal women who can produce more potent metabolites. In our randomized-controlled study, soy-induced changes of bone turnover markers were assessed in healthy, menstruating women after dividing them into subgroups according to metabolizing phenotypes.
Sixty healthy premenopausal female volunteers of Korean ethnicity between the ages of 30 and 50 yr (37.2±4.8 yr, mean±SD) were recruited to participate in this study. Throughout the study, subjects had no restrictions and continued their usual activities at home and at work. Informed consent was obtained from each study participant and this study was approved by the Institutional Review Board of the Cheil General Hospital and Women's Healthcare Center.
This study was designed as a randomized, double-blind, placebo-controlled study. The participants were randomly assigned to receive either isoflavone capsules (soy group; 120 mg/capsule, n=32) or capsules containing the same dose of lactose as a placebo (n=21) once a day for three menstrual cycles. Four of the 36 subjects in the soy group dropped out because of side effects (breast pain and weight gain). Three of the 24 placebo subjects dropped out for different reasons; one subject due to pregnancy, and two subjects due to failure of follow-up. All subjects consumed their habitual diets with detailed instructions to minimize phytoestrogen consumption. Food consumption was recorded at the beginning and end of the study. The soy extract isoflavone in 120 mg/capsules (Rexgene Biotech Co., Cheongwon, Korea) consisted of 3.68% daidzin, 13.97% genistin, and 18.51% glycitein. Baseline blood samples and 24-hr urine collections were obtained from Day 3 to Day 6 of each menstrual cycle, and follow-up samples after intervention were collected on the same day of the menstrual cycle as the day when baseline samples were collected. Fasting blood and 24-hr urine samples were also collected, and the serum was prepared immediately and frozen at -70℃ until it was assayed.
Serum osteocalcin (OC) levels were determined by Radio Immunoassay (RIA) using an Osteocalcin 125I RIA kit (Incstar Corp., Stillwater, MN, U.S.A.; intra- and interassay CV, 7.8% and 9.2%, respectively). Urine deoxypyridinoline (DPD) was assayed by the enzyme-linked immuno-sorbent assay (ELISA) method (Osteomark, Ostex, Seattle, WA, U.S.A.; intra- and interassay CV, 5.7% and 3.5%, respectively) and corrected for creatinine. Serum 17β-estradiol (E2) was assayed via RIA (Diagnostic System laboratories, Webstar, TX, U.S.A.; CV=11%). Serum lutenizing hormone (LH) and follicular stimulating hormone (FSH) levels were measured by immunoradiometric assays (Diagnostic Products Corp.; CV, 14% for LH and 11% for FSH).
Twenty-four hour urine samples were analyzed for phytoestrogens using solid-phase extraction (SPE) followed by HPLC and tandem mass spectrometry. Conjugated analytes in urine were hydrolyzed by the addition of β-glucuronidase/sulfatase (Helix pomatia, H-1, Sigma Chemical, St. Louis, MO, U.S.A.) and incubated overnight at 37℃. Deconjugated samples were spiked with an internal standard (flavone, Sigma Chemical) and then extracted with Oasis hydrophilic-lipophilic balance (HLB) solid-phase extraction (SPE) (30 mg HLB, 1 mL, Waters Corporation, Milford, MA, U.S.A.). The phytoestrogens were separated by a reversed-phase high-performance liquid chromatography (HPLC) (150×1.0 mm Alltima column, Alltech, Deerfield, IL, USA) and a flow rate of 50 µL/min (0.1% formic acid, 25% MeOH, 25% acetonitrile). All analytes were measured by tandem mass spectrometry (API 2000, MDS/Sciex, Concord, Ontario, Canada) using electrospray ionization (ESI) in the positive-ion multiple-reaction monitoring mode. The intra- and interassay CVs were 7% and 10%, respectively.
Statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS, Inc., Chicago, IL, U.S.A.). Data were evaluated using a one-way analysis of variance (ANOVA) and a paired two-tailed t test. To assess interrelationships between variables, Spearman's correlation coefficient analyses were also used. In the case of significant differences, a non-parametric test was used to further specify these differences. P values below 0.05 (paired two-tailed t test) were considered significant.
The clinical characteristics of study subjects are summarized in Table 1. Three of the 24 placebo subjects and four of the 36 subjects in the soy group dropped out because of difficulties complying with the study protocol. Therefore, we included 21 women in the placebo group and 32 women in the soy group for the final data analyses. At baseline, there were no significant differences in age, body mass index (BMI), food intake, bone markers, or hormone status between the soy and placebo groups. Urinary isoflavones and their metabolites were detected in all subjects at baseline. No significant differences could be found in the baseline excretion concentrations between the two groups (Fig. 2).
After a soy challenge during three menstrual cycles, the daily urinary excretion of all measured isoflavone metabolites was significantly increased in the soy group, while significant changes were not observed in the placebo group except for the excretion of dihydrogenistein (DGTN), which showed only a slight but statistically significant incremental increase at the follow-up (Fig. 2). The marked inter-individual variation was observed in the urinary excretion of isoflavone metabolites after soy intake (Fig. 3). Individual variations of DZ and GTN after a challenge exceeded normal levels by 100-fold and 400-fold, respectively. EQL also had a high individual difference in its excretion, exceeding normal levels by 400-fold. Urinary excretion of DZ was highly correlated with GTN, dihydrodaidaein (DDZ) and O-DMA, but not with EQL. The urinary concentration of EQL did not show any significant correlations with any other metabolites (Fig. 5).
Significant changes of circulating hormones, including LH, FSH, and estradiol (E2), were not found after a challenge in both groups (data not shown). Body weight and BMI did not change significantly after follow-up in both groups (data not shown). However, the serum concentrations of OC (the bone formation marker) increased significantly after a challenge in the soy group but not in the placebo group (Fig. 4). Of the subjects in the soy group, eight women (25%) could be categorized as EQL high-excretors (>1,000 nM/24 hr) (17). EQL-excretors showed a tendency for an increase in OC. We recategorized subjects according to the excretion of GTN, because GTN also has the strongest ER-binding affinities and estrogenic activities among all the metabolites (4, 29). When the GTN high-excretors were defined arbitrarily as women whose urinary excretion of GTN was greater than 2,000 nM/day, 15 women were included in the GTN high-excretor group. All DZ metabolites after intervention were significantly higher in GTN high-excretors compared with the placebo group and the GTN low-excretors (data not shown). Daily excretion of DZ was over 2,500 nM/day in all GTN high-excretors. Seven of the eight women who were categorized as EQL excretors could be also categorized as GTN high-excretors. Basal characteristics including age, weight, BMI, and sex hormone levels were not different among these subgroups. Serum OC concentrations in the GTN high-excretors increased significantly after the challenge but did not increase in either the placebo group or the GTN low-excretors (Fig. 6).
The bone-resorption marker, urine DPD, was decreased after a challenge in both the placebo and soy groups (Fig. 4). A decrement of DPD levels after a challenge, however, was not observed in either the EQL excretors or the GTN high-excretors (data not shown). When we subdivided women by the excretion levels of O-DMA, the weak metabolite of DZ, any tendency for soy-induced increments in bone remodeling could not be found in the O-DMA high-excretors (data not shown).
Our results indicate that the high ingestion of isoflavones increases bone turnover in the early follicular phase of menstruation. This antiestrogenic action is observed prominently in women who can produce and absorb the metabolites with relatively strong binding affinities to ERs. We also noted a marked inter-individual variation after a soy challenge in the excretion of isoflavone metabolites including the intermediate metabolites of GTN and DZ. The personal variation of daily urinary excretion of metabolites exceeds several hundred-fold after the soy challenge. The difference between minimum and maximum was over 2,000-fold in the excretory levels of some intermediate metabolites such as DDZ and DGTN.
Isoflavones in soy proteins are conjugated to sugars. These β-glucosides, including daidzin and genistin, are biologically inactive and can be absorbed only after being hydrolyzed into the aglycones, DZ and GTN, respectively (1, 2, 15-17). Hydrolysis is known to be an extremely efficient process, occurring along the entire length of the intestine by the action of both the brush border membrane and the bacterial β-glucosidase. In our study, a broad range of metabolites could be detected in urine after a challenge, which is in agreement with previous studies (15-17). A proportion of DZ and GTN appeared to escape further metabolism within the intestine, being absorbed as aglycones just after hydrolysis by the glucosidase and largely cleared in the urine. The remnants underwent more intestinal fermentation via the bacterial reaction that takes place distally and presumably in the colon, yielding various intermediate and end products, which are also readily absorbed and then largely cleared in urine. Interestingly, in our study the tested metabolites of DZ were highly correlated with each other, except for EQL. GTN also showed a significant correlation with its metabolite DGTN and even with other DZ metabolites except for EQL. This finding suggests that DZ and GTN undergo a similar metabolism using the same metabolizing enzymes to produce O-DMA or DGTN, respectively. EQL, however, seems to have a distinct metabolic pathway using different enzymes from different intestinal bacteria strains. It has been demonstrated that the formation of EQL is exclusively dependent on intestinal microflora. The lack of EQL in germ-free animals and infants fed infant formula revealed the need for an active microflora for its formation (18-20). The repeated administration of isoflavones to the same adults showed consistently that those who are "EQL excretors" seem to remain "EQL excretors" over time (21). Three strains of bacteria responsible for EQL formation have been identified in Japanese subjects by in vitro culturing of fecal flora (14).
In approximately one-fourth of our study subjects, all of the tested isoflavones were excreted in concentrations of less than 500 nM/day even though the participants were challenged daily with a large amount of soy extract. The reasons why some people do not have an ability to excrete enough isoflavonoids after a challenge are unclear. Some studies have shown that some dietary components could alter the intestinal environment and affect the metabolism of isoflavones, but the data are still controversial (14). Studies on the prevalence of poor excretors have not been conducted yet.
Our study shows that the high consumption of isoflavones can affect physiological functions of bone remodeling in premenopausal women. To our knowledge, only two small studies have been reported to evaluate the effects of isoflavones on bone turnover during menstrual cycles. In a randomized, cross-over study with 14 young, menstruating women, the daily consumption of 65 mg and 130 mg of soy isoflavones increased the bone-resorption marker, urine DPD, at the early follicular phase after three menstrual cycles (22). In another study of a randomized, cross-over design, after daily ingestion of 52 mg isoflavones for more than one menstrual cycle, 14 premenopausal women showed a significant increase in tartrate-resistant acid phosphatase (TRAP) compared with the placebo group, but not in another bone-resorption marker, C-telopeptide (CTx) (23). However, no significant effects of soy-intervention were observed for the bone-formation marker OC in both studies. The discrepancy between our study and the previous data on the bone-formation marker is not clear. The failure of OC to show a significant, soy-induced change in previous studies may be due to the heterogeneity of study subjects, many of whom might be low excretors of additional estrogenic isoflavones, such as EQL or GTN.
After soy-induction, we could not find any significant changes of circulating hormones including serum E2, LH, and FSH. However, some intervention studies have shown decreased estrogen levels and changes in menstrual-cycle length after increased soy intake (24, 25). In the intervention study from California, the soy-induced estrogen-lowering effect was restricted to Asian women (26). Other intervention studies have failed to observe any significant effect of isoflavones on sex hormones and menstrual patterns (27). In a previous study, five premenopausal EQL excretors had lower concentrations of estrone and estron-sulfate and higher sex hormone-binding globulin than nine EQL non-excretors in certain phases of the menstrual cycle including the early follicular phase. Serum E2, however, did not show any significant differences between the subgroups (28). Most of the reported data are not at the proper scale to adjust for a large number of unmeasured factors that may influence the clinical effects of soy. Therefore, the mechanism of a soy-induced antiestrogenic effect on premenopausal bone remodeling is still unclear; it may be an indirect effect caused by the changes of circulating sex hormones or a direct effect on bone. Isoflavones can bind both types of ER, especially ERβ, and exhibit weak estrogenic activity. The antiestrogenic activity may be partially explained by its competition with endogenous E2 for ERs and then attenuating estrogenic action by their lower intrinsic activity.
EQL has the strongest estrogenic properties among DZ metabolites. GTN has been shown to be similar or even stronger than EQL in ER-binding affinities and estrogenic activities (4, 29). In our study, a significant increment of bone turnover could be observed more prominently in women whose urinary GTN excretion was more than 2,000 nM/day after soy-intervention, rather than in EQL excretors. Since most women who were categorized as GTN high-excretors could also excrete high concentrations of DZ and its metabolites including even EQL, the GTN effect in our study may reflect a sum effect including other isoflavone metabolites. A recently published, large-scale clinical trial, however, has shown a GTN-induced positive effect on BMD in postmenopausal women; GTN can independently increase BMD at both the lumbar and femoral neck areas by means of a two-year treatment of daily doses of 54 mg of GTN (7).
Although most reported studies are small-scale and do not contain enough participants to confirm the skeletal effect of isoflavones especially on premenopausal bone remodeling, the previous data are consistent with our results; isoflavones can modulate the bone turnover rate and may act as estrogen antagonists in certain phases of the menstrual cycle. Furthermore, our results identified the importance of the awareness that a large degree of individual variation exists in the metabolism and biological functions of phytoestrogens. Since the magnitude of the effect of isoflavones on bone turnover is quite small despite relatively high doses of isoflavones, their clinical importance should be clarified by further, large-scale studies investigating the long-term effects on BMD according to the metabolic phenotype.
Figures and Tables
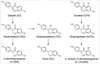 | Fig. 1Metabolic pathway of the major soy isoflavones daidzin and genistin. They are cleaved by intestinal glucosidases to the aglycones, daidzein (DZ) and genistein (GTN). DZ and GTN are further metabolized by intestinal microflora into several metabolites: dihydrogenistein (DGTN), dihydrodaidzein (DDZ), tetrahydrodaidzein (TDZ), o-desmethylangolensin (O-DMA), and equol (EQL). |
 | Fig. 2Mean (±SEM) urinary excretion of isoflavones and their metabolites before and after a soy challenge in the placebo (left) and soy group (right). *, †, ‡Significantly different from baseline: *P<0.05; †P<0.01; ‡P<0.001 (paired two-tailed t test). |
 | Fig. 3Urinary equol (EQL), o-desmethangiolensin (O-DMA), dihydrodaidzein (DDZ), dihydrogenistein (DGTN), genistein (GTN), and daidzein (DZ) excretion in 32 subjects in the soy group after a soy challenge. |
 | Fig. 4Changes of serum osteocalcin (OC), a bone-formation marker, and urine deoxypyridinoline (DPD), a bone-resorption marker, before and after a soy challenge in the placebo and the soy group (paired two-tailed t test). |
 | Fig. 5Scatter plots between urinary concentrations of isoflavonoid metabolites. Daidzein (DZ) was significantly correlated with its metabolites, except for equol (EQL) (upper three and lower first plots). EQL was not correlated with any other metabolites (lower panel) (r=Pearson correlation, P=significance by two-tailed Z test). |
 | Fig. 6Changes of serum osteocalcin (OC) before and after the challenge in subgroups divided by several metabolic phenotypes. Eight women (25%) were categorized as EQL excretors (>1,000 nM/day). The GTN high-excretors were defined arbitrarily as women whose urinary excretion of GTN was greater than 2,000 nM/day. |
References
1. Ososki AL, Kennelly EJ. Phytoestrogens: a review of the present state of research. Phytother Res. 2003. 17:845–869.

2. Murkies AL, Wilcox G, Davis SR. Clinical review 92: phytoestrogens. J Clin Endocrinol Metab. 1998. 83:297–303.
3. Harris DM, Besselink E, Henning SM, Go VLW, Heber D. Phytoestrogens induce differential estrogen receptor alpha- or beta-mediated responses in transfected breast cancer cells. Exp Biol Med (Maywood). 2005. 230:558–568.

4. Hwang CS, Kwak HS, Lim HJ, Lee SH, Kang YS, Choe TB, Hur HG, Han KO. Isoflavone metabolites and their in vitro dual functions: they can act as an estrogenic agonist or antagonist depending on the estrogen concentration. J Steroid Biochem Mol Biol. 2006. 101:246–253.

5. Potter SM, Baum JA, Teng H, Stillman RJ, Shay NF, Erdman JW Jr. Soy protein and isoflavones: their effects on blood lipids and bone density in postmenopausal women. Am J Clin Nutr. 1988. 68(6):Suppl. 1375S–1379S.

6. Alekel DL, Germain AS, Peterson CT, Hanson KB, Stewart JW, Toda T. Isoflavone-rich soy protein isolate attenuates bone loss in the lumbar spine of perimenopausal women. Am J Clin Nutr. 2000. 72:844–852.

7. Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, Lubrano C, Bonaiuto M, D'Anna R, Cannata ML, Corrado F, Adamo EB, Wilson S, Squadrito F. Effects of the phytoestrogen genistein on bone metabolism in osteopenic postmenopausal women: a randomized trial. Ann Intern Med. 2007. 146:839–847.
8. Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, D'Anna R, Corrado F, Pizzoleo MA, Cincotta M, Altavilla D, Ientile R, Squadrito F. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002. 17:1904–1912.

9. Cheong JM, Martin BR, Jackson GS, Elmore D, McCabe GP, Nolan JR, Barnes S, Peacock M, Weaver CM. Soy isoflavones do not affect bone resorption in postmenopausal women: a dose-response study using a novel approach with 41Ca. J Clin Endocrinol Metab. 2007. 92:577–582.

10. Reinwald S, Weaver CM. Soy isoflavones and bone health: a double-edged sword? J Nat Prod. 2006. 69:450–459.

11. Ho SC, Chan SG, Yi Q, Wong E, Leung PC. Soy intake and the maintenance of peak bone mass in Hong Kong Chinese women. J Bone Miner Res. 2001. 16:1363–1369.

12. Anderson JJ, Chen X, Boass A, Symons M, Kohlmeier M, Renner JB, Garner SC. Soy isoflavones: no effects on bone mineral content and bone mineral density in healthy, menstruating young adult women after one year. J Am Coll Nutr. 2002. 21:388–393.

13. Mei J, Yeung SS, Kung AW. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001. 86:5217–5221.

14. Setchell KD, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002. 132:3577–3584.

15. Joannou GE, Kelly GE, Reeder AY, Waring M, Nelson C. A urinary profile study of dietary phytoestrogens. The identification and mode of metabolism of new isoflavonoids. J Steroid Biochem Mol Biol. 1995. 54:167–184.

16. Chang YC, Nair MG. Metabolites of daidzein and genistein by intestinal bacteria. J Nat Prod. 1995. 58:1892–1896.
17. Rowland I, Wiseman H, Sanders T, Adlercreutz H, Bowey E. Metabolism of oestrogens and phytoestrogens: role of the gut microflora. Biochem Soc Trans. 1999. 27:304–308.

18. Axelson M, Setchell KD. The excretion of lignans in rats-evidence for an intestinal bacterial source for this new group of compounds. FEBS Lett. 1981. 123:337–342.
19. Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Exposure of infants to phyto-oestrogens from soy-based infant formula. Lancet. 1997. 350:23–27.

20. Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998. 68(6):Suppl. 1453S–1461S.

21. Setchell KD, Brown NM, Desai PB, Desai PB, Zimmer-Nechimias L, Wolfe B, Jakate AS, Creutzinger V, Heubi JE. Bioavailability, disposition, and dose-response effects of soy isoflavones when consumed by healthy women at physiologically typical dietary intakes. J Nutr. 2003. 133:1027–1035.

22. Wangen KE, Duncan AM, Merz-Demlow BE, Xu X, Marcus R, Phipps WR, Kurzer MS. Effects of soy isoflavones on markers of bone turnover in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 2000. 85:3043–3048.

23. Zittermann A, Geppert J, Baier S, Zehn N, Gouni-Berthold I, Berthold HK, Reinsberg J, Stehle P. Short-term effects of high soy supplementation on sex hormones, bone markers, and lipid parameters in young female adults. Eur J Nutr. 2004. 43:100–108.

24. Cassidy A, Bingham S, Setchell KD. Biological effects of a diet of soy protein rich in isoflavones on the menstrual cycle of premenopausal women. Am J Clin Nutr. 1994. 60:333–340.

25. Lu LJ, Anderson KE, Grady JJ, Nagamani M. Effects of soya consumption for one month on steroid hormones in premenopausal women: implication for breast cancer risk reduction. Cancer Epidemiol Biomarkers Prev. 1996. 5:63–70.
26. Wu AH, Stanczyk FZ, Hendrich S, Murphy PA, Zhang C, Wan P, Pike MC. Effects of soy foods on ovarian function in premenopausal women. Br J Cancer. 2000. 82:1879–1886.

27. Maskarinec G, Franke AA, Williams AE, Hebshi S, Oshiro C, Murphy S, Stanczyk FZ. Effects of a 2-year randomized soy intervention on sex hormone levels in premenopausal women. Cancer Epidemiol Biomarkers Prev. 2004. 13:1736–1744.
28. Duncan AM, Merz-Demlow BE, Xu X, Phipps WR, Kurzer MS. Premenopausal equol excretors show plasma hormone profiles associated with lowered risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2000. 9:581–586.
29. Kuiper GG, Lemmen JG, Carlsson B, Corton C, Safe SH, van der Saag PT, van der Burg B, Gustafsson JÅ. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998. 139:4252–4263.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download