Abstract
Unilateral obstruction or injury to the vas deferens can result in significant injury to the contralateral testicle. Although various pathways have been proposed, the mechanism of contralateral testicular deterioration remains controversial. The present animal study was performed to evaluate the effects of unilateral vasectomy on ipsilateral and contralateral testicular histology and fertility in rats that were chemically sympathectomized neonatally. The study comprised 40 male albino rats: 20 received a placebo and the other 20 underwent chemical sympathectomy neonatally. When 60 days old, each group of 20 rats was divided into two groups that underwent either a sham operation or an operation to create unilateral left vasectomy. Eight weeks after surgery, each male rat was housed with two known fertile female rats for 25 days, and then their testes were harvested. Mean seminiferous tubular diameters (MSTD) and mean testicular biopsy scores (MTBS) were determined for each testis. Although MSTD and MTBS were not significantly different between groups, chemical sympathectomy prevented the decrease in total fertility rates of the rats with unilateral left vasectomy in our study. Prevention of this decrease by chemical sympathectomy suggests that the sympathetic nervous system may play a role in the testicular degeneration associated with vasectomy.
Unilateral obstruction or injury to the vas deferens can result in significant injury to the contralateral testicle (1-3). Although studies have indicated that the damage can be reversed by repair of the obstructed vas deferens (4), other studies have suggested that the effects are irreversible (2). Although various pathways have been proposed, the mechanism of contralateral testicular deterioration remains controversial. The most popular theory, commonly proposed to explain the contralateral testicular damage, has been an autoimmune response (3, 5). Currently, the probable mechanism of contralateral injury is accepted as a vasospasm through a sympathetic reflex arc, and hypoxia may be the reason for contralateral injury after unilateral testicular obstruction (6, 7). Succeeding studies have shown decreases in testicular oxygen content and adenylate energy charge, and an increase in biochemical indicators of tissue hypoxia (8, 9). Chemical sympathectomy reduced the increase in biochemical indicators of tissue hypoxia and preserved spermatogenetic function (10-12). These findings suggested that contralateral testicular damage resulted from a reflex vasospasm preceded by sympathetic activation. Thus, the present animal study was performed to evaluate the effects of unilateral vasectomy on ipsilateral and contralateral testicular histology and fertility in rats that were chemically sympathectomized neonatally.
The study comprised 40 male albino rats: 20 received a placebo and the other 20 underwent chemical sympathectomy neonatally. The experimental protocol was approved by the Animal Ethics Committee of Akdeniz University, Turkey. The animals were handled according to internationally accepted principles for care of laboratory animals. The rats were maintained under standard laboratory conditions with a 12: 12 hr light:dark cycle, with free access to food pellets and tap water. They were separated equally into the four groups: Group 1: a sham (control) group that received a placebo; Group 2: a group with sham that received 6-hydroxydopamine hydrobromide; Group 3: a group with unilateral left vasectomy that received a placebo; Group 4: a group with unilateral left vasectomy that received 6-hydroxydopamine hydrobromide.
Within 24 hr of birth, 20 rats each received an intraperitoneal injection with placebo (physiological saline solution 0.2 mL/day, for 7 days) or 6-hydroxydopamine hydrobromide solution 0.075 mg/g, for 7 days. Dopamine solutions were prepared each day by dilution in physiological saline containing 0.1 mg/mL of ascorbic acid. When 60 days old, each group of 20 rats was divided into two groups that underwent either a control operation or an operation to create unilateral left vasectomy. To create a unilateral abdominal testis, a left inguinoscrotal incision was made in the abdomen, and the left vas deferens was retrieved and exposed. Using 4.0 silk suture, the vas deferens was serially ligated and subsequently transected between the ligatures. Each end was then replaced within the abdominal cavity. The incision was closed in 2 layers with 4.0 silk suture, in a running fashion. The procedure was duplicated for the sham animals. Briefly, the incision was made, vasa deferentia were exposed, 4.0 silk was passed, but the vasa deferentia were not ligated or transected. Eight weeks after surgery, each male rat was housed with two known fertile female rats for 25 days, and then their testes were harvested.
Specimens were fixed in Bouin's solution and embedded in paraffin blocks. Sections were cut and stained with hematoxylin-eosin, periodic acid-Schiff (PAS) and Masson's trichrome. Testicular biopsies were examined in random order by a pathologist unaware of the treatments. The 50 most circular tubules were identified in each testicular biopsy section and their diameters were measured using a ×40 objective and an ocular micrometer. The testicles were evaluated histologically with respect to the following characteristics: 1) seminiferous tubular diameter; 2) morphology and progression of maturation of the germinal epithelium; and 3) morphology of the tunica propria and interstitial components, specifically to determine whether fibrosis, hyalinization, or an inflammatory infiltrate could be identified. The mean seminiferous tubular diameter (MSTD) was determined for each testis. Germinal epithelial maturity was graded using a modified Johnsen testicular biopsy score (13); using a ×40 objective, 50 tubules were evaluated and each tubule was given a score from 1 to 10. The mean testicular biopsy score (MTBS) was determined for each testis. The female rats were observed for an additional 25 days. The number of rats impregnated by each male rat and the number of rats delivered by each female rat were recorded.
The statistical comparisons between MSTD and MTBS values of the groups were performed using multi-way analysis of variance. Total fertility rates of the groups were compared using t-test, with P<0.05 taken to indicate significant differences; all values are presented as the mean (SD).
A total of 6 rats died during study (1 from group 1, 1 from group 2, 1 from group 3, and 3 from group 4). There was no statistically significant difference in histological examination between any of the groups (P>0.05).
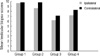
The MTBS of the ipsilateral and contralateral testes were 9.67±0.50 and 9.78±0.44 in Group 1, 8.67±0.5 and 9.78±0.42 in Group 2, 6.22±0.44 and 7.11±0.33 in Group 3, and 7.22±0.43 and 8.29±0.48 in Group 4, respectively (P>0.05; Fig. 1).
While the MSTD of the ipsilateral and contralateral testes did not differ significantly between groups, those of the ipsilateral testes of all of the groups were lower than in the corresponding contralateral testes. The MSTD of the ipsilateral and contralateral testes were 242.666±89.326 and 249.906±75.907 in Group 1, 178.013±38.392 and 209.673±104.931 in Group 2, 147.113±6.667 and 155.893±12.954 in Group 3, and 165.440±15.449 and 188.140±23.360 in Group 4, respectively (P>0.05; Fig. 2).
Total fertility rates of each group are shown in Table 1. These rates were 88.8% in Group 1, 66.6% in Group 2, 77.7% in Group 3, and 85.7% in Group 4. Total fertility rate of Group 4 was significantly higher than that in Group 3 (P<0.05).
Unilateral obstruction of the vas deferens can occur for a variety of reasons, including epididymitis, trauma, or iatrogenic damage to the vas during hydrocele repair, orchidopexy, or inguinal herniorrhaphy (14). Unilateral injury to the vas deferens has been associated with damage to the contralateral testicle, and this damage is thought to be mediated through antisperm autoantibodies (3, 5). A current theory of contralateral testicular injury proposes a reflexive decrease in contralateral testicular blood flow and an increase in the biochemical indicators of tissue hypoxia within the contralateral testes (6, 7). The mechanism by which contralateral testicular blood flow is affected after unilateral vas deferens obstruction has not been thoroughly evaluated. The vasospasm in the contralateral testis was suggested to arise by a neurovascular pathway triggered by an ipsilateral testicular stimulus, running through a sympathetic reflex arc, resulting in decreased blood flow (15). Ipsilateral vas deferens obstruction also resulted in increased levels of lactic acid and hypoxanthine in both testes. Decreases in the blood flow of both testes and oxidative stress have been reported after ipsilateral efferent duct ligation (16). Ligation of the vas deferens should increase the pressure within the distal structures and the structure of the tunica albuginea may not allow for expansion to decrease the pressure. The increased pressure may therefore decrease the blood flow to the ipsilateral testis through increasing the intratesticular pressure. The increases in the levels of lactic acid and hypoxanthine within the contralateral testis suggest a reflex decrease in contralateral testicular blood flow when the ipsilateral testis is under stress after ligation of the ipsilateral vas deferens. Andiran et al. (1) reported that unilateral testicular torsion, unilateral vas deferens obstruction, unilateral abdominal testis, and unilateral venous obstruction not only damage the ipsilateral but also the contralateral testes. As the lactic acid and hypoxanthine levels within the contralateral testis were greater than in the controls, testicular torsion and vas deferens obstruction seem to share a common pathway, which may be a reflex decrease in contralateral testicular blood flow, for their effects on the contralateral testis (1). An effective circulatory system in the testis is the most critical factor for functional spermatogenesis (17).
The increase in biochemical indicators of tissue hypoxia and contralateral testicular damage can be prevented by chemical sympathectomy (10, 11, 18). Experimentally, the sympathetic nervous system was also activated by partial ligation of the left renal vein in the adult rat, which was blocked by chemical sympathectomy (19). The evaluation of contralateral testis through the model of testicular torsion has revealed a decrease in blood flow and an increase in factors associated with tissue hypoxia, e.g., lactic acid and hypoxanthine; chemical sympathectomy reduced the effects of tissue hypoxia and preserved testicular histology, fertility and fecundity (9, 10). Additionally, unilateral undescent has also been reported to result in elevated levels of the products of tissue hypoxia in the contralateral testis (20). Karnak et al. (18) evaluated the effects of abdominal testis on ipsilateral and contralateral testicular histology, fertility and fecundity in rats that were chemically sympathectomized neonatally. In their investigation, although fertility and fecundity rates and MTBSs were not significantly different, chemical sympathectomy prevented the decrease in MSTDs that occurred in the contralateral testis. These findings suggested that the sympathetic system may play a role in contralateral testicular deterioration. Therefore, we evaluated the effects of unilateral vasectomy on ipsilateral and contralateral testicular histology and fertility in rats that were chemically sympathectomized neonatally. Although MSTD and MTBS were not significantly different between groups, chemical sympathectomy prevented the decrease in total fertility rates of the rats with unilateral left vasectomy in our study. Prevention of this decrease by chemical sympathectomy suggests the sympathetic nervous system may play a role in the testicular degeneration associated with vasectomy.
Figures and Tables
Fig. 1
Mean testicular biopsy scores (MTBS) in the ipsilateral and contralateral testes in each group.
ACKNOWLEDGMENT
The authors wish to thank the Pathology Department for histological evaluation of testes.
References
1. Andiran F, Okur DH, Kilinc A, Gedikoglu G, Kilinc K, Tanyel FC. Do experimentally induced ipsilateral testicular torsion, vas deferens obstruction, intra-abdominal testis or venous obstruction damage the contralateral testis through a common mechanism? BJU Int. 2000. 85:330–335.

2. West DA, Chehval MJ, Winkelmann T, Martin SA. Effect of vasovasostomy on contralateral testicular damage associated with unilateral vasectomy in mature and immature Lewis rats. Fertil Steril. 2000. 73:238–241.

3. Chehval MJ, Martin SA, Alexander NJ, Winkelmann T. The effect of unilateral injury to the vas deferens on the contralateral testis in immature and adult rats. J Urol. 1995. 153:1313–1315.

4. Matsuda T, Horii Y, Yoshida O. Unilateral obstruction of the vas deferens caused by childhood inguinal herniorrhaphy in male infertility patients. Fertil Steril. 1992. 58:609–613.

5. Chakraborty J, Hikim AP, Jhunjhunwala JS. Quantitative evaluation of testicular biopsies from men with unilateral torsion of spermatic cord. Urology. 1985. 25:145–150.

6. Dokucu AI, Ozturk H, Ozdemir E, Ketani A, Buyukbayram H, Yucesan S. The protective effects of nitric oxide on the contralateral testis in prepubertal rats with unilateral testicular torsion. BJU Int. 2000. 85:767–771.

7. Tanyel FC, Buyukpamukcu N, Hicsonmez A. Contralateral testicular blood flow during unilateral testicular torsion. Br J Urol. 1989. 63:522–524.

8. Salman AB, Mutlu S, Iskit AB, Guc MO, Mutlu M, Tanyel FC. Hemodynamic monitoring of the contralateral testis during unilateral testicular torsion describes the mechanism of damage. Eur Urol. 1998. 33:576–580.

9. Akgur FM, Kilinc K, Tanyel FC, Buyukpamukcu N, Hicsonmez A. Ipsilateral and contralateral testicular biochemical acute changes after unilateral testicular torsion and detorsion. Urology. 1994. 44:413–418.

10. Karaguzel G, Tanyel FC, Kilinc K, Buyukpamukcu N, Hicsonmez A. The preventive role of chemical sympathectomy on contralateral testicular hypoxic parameters encountered during unilateral testicular torsion. Br J Urol. 1994. 74:507–510.
11. Karaguzel G, Gedikoglu G, Tanyel FC, Buyukpamukcu N, Hicsonmez A. Subsequent biological effects of chemical sympathectomy in rats undergoing unilateral testicular torsion. Eur Urol. 1995. 28:147–151.
12. Oguzkurt P, Okur DH, Tanyel FC, Buyukpamukcu N, Hicsonmez A. The effects of vasodilatation and chemical sympathectomy on spermatogenesis after unilateral testicular torsion: a flow cytometric DNA analysis. Br J Urol. 1998. 82:104–108.

13. Coskun S, Tbakhi A, Jaroudi KA, Uzumcu M, Merdad TA, Al-Hussein KA. Flow cytometric ploidy analysis of testicular biopsies from sperm-negative wet preparations. Hum Reprod. 2002. 17:977–983.

15. Ciftci AO, Muftuoglu S, Cakar N, Tanyel FC. Histological evidence of decreased contralateral testicular blood flow during ipsilateral testicular torsion. Br J Urol. 1997. 80:783–786.

16. Zini A, Schlegel PN. Cu/Zn superoxide dismutase, catalase and glutathione peroxidase mRNA expression in the rat testis after surgical cryptorchidism and efferent duct ligation. J Urol. 1997. 158:659–663.

17. Free MJ, Jaffe RA. Dynamics of circulation in the testis of the conscious rat. Am J Physiol. 1972. 223:241–248.

18. Karnak I, Gedikoglu G, Tanyel FC, Buyukpamukcu N, Hicsonmez A. The effects of chemical sympathectomy on contralateral testicular histology, fertility and fecundity in unilateral abdominal testes. Br J Urol. 1996. 77:580–584.

19. Ozturk H, Tander B, Aydin A, Okumus Z, Cetinkursun S. The effects of chemical sympathectomy on testicular injury in varicocele. BJU Int. 2001. 87:232–234.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download