Abstract
We report a very rare case of hemangioblastomatosis that developed after surgical removal of a solitary cerebellar hemangioblastoma (HB). A 51-yr-old man presented with back pain 10 yr after undergoing surgery for cerebellar HB. Magnetic resonance imaging showed numerous mass lesions along the entire neuraxis accompanied by prominent leptomeningeal enhancement. Genomic DNA analysis showed no mutation in the von Hippel-Lindau (VHL) genes. A surgical specimen obtained from a lesion in the cauda equina showed pathological findings identical to those of the cerebellar HB that had been resected 10 yr earlier. External beam radiation therapy and radiosurgery were subsequently performed; however, the patient succumbed one year after receiving the diagnosis of hemangioblastomatosis. The reduction of tumor cell spillage during surgery and regular long-term follow-up are recommended for patients with HBs.
Hemangioblastoma (HB) of the central nervous system is a benign neoplasm of the central nervous system that usually involves the brain and spinal cord. It may occur sporadically or in association with von Hippel-Lindau (VHL) disease (1-5). In sporadic, non-VHL cases, the tumors are usually solitary, and local recurrences are seen occasionally after surgery (1, 2, 5). In contrast, HBs associated with VHL disease are usually multiple and continue to arise over the course of a patient's life, which necessitates lifelong surveillance (2-5, 7). Disseminated HB is an extremely unusual type of recurrence, and it has recently been called hemangioblastomatosis (10-16). Hemangioblastomatosis is very rare and has been reported in patients with or without VHL. We report a case of hemangioblastomatosis in a patient without VHL and discuss the biological and clinical features associated with this disease.
In 1996, a 41-yr-old man underwent surgical excision of a solitary cerebellar mass with the pathological diagnosis of HB (Fig. 1A-C). He had no family history or clinical stigmata to suggest the presence of VHL disease. Gross total removal of the tumor was possible, and no evidence of residual or recurrent disease was observed on magnetic resonance imaging (MRI) performed 1 yr after the surgery (Fig. 1D). Ten years after the surgery, he returned to the hospital complaining of low back pain and hypesthesia in his right posterior thigh. MRI of the brain showed multiple mass lesions in the suprasellar area and in the anterior aspect of the upper spinal cord accompanied by diffuse leptomeningeal enhancement (Fig. 2A). Whole-spine MRI revealed numerous tiny enhancing nodules suggestive of leptomeningeal metastasis along the spinal cord and cauda equina (Fig. 2B). No retinal lesion was found on examination of the fundus. Computed tomography (CT) of the abdomen showed no abnormal findings. A blood sample was collected from the patient after informed consent was obtained. Genomic DNA was isolated from peripheral blood leukocytes using a Wizard genomic DNA purification kit according to the manufacturer's instructions (Promega, Madison, WI, U.S.A.). The three exons of the VHL gene as well as their flanking introns were amplified and sequenced by an ABI 3730 sequencer (AME Bioscience, Toroed, Norway). Mutation was not found. The patient underwent surgical resection of the cauda equina lesion and subtotal removal of the tumor. Histopathological examination confirmed the diagnosis of HB, and the microscopic findings were identical to those of the cerebellar HB operated on 10 yr earlier (Fig. 2C). He subsequently received fractionated radiotherapy (a total dose of 3,600 cGy) for a residual lesion in the cauda equina. Brain MRI scans obtained 6 months after the diagnosis of recurrence revealed interval growth of the suprasellar lesion, and gamma knife radiosurgery was performed. He also underwent ventriculoperitoneal shunt due to progressive hydrocephalus. Although the treated lesions showed no further growth, his condition gradually deteriorated with general weakness, anorexia, and frequent vomiting. The patient died of septic shock and respiratory failure at 1 yr after the diagnosis of disseminated recurrence.
HBs account for 1 to 2% of primary intracranial tumors and 5 to 15% of posterior fossa tumors in adults (2-5, 7, 8). Approximately 62% of HBs arise sporadically, and 38% occur in association with VHL disease (6). The average age at presentation of sporadic cerebellar HBs is approximately 35 yr, and these tumors are more common in men. HB is thought to be a benign tumor curable by microsurgery; however, several previous studies have reported that the recurrence rate after surgical excision is 15-27% (9).
VHL disease is an autosomal-dominant neoplasia syndrome caused by a germline mutation or deletion on the short arm of chromosome 3. Patients with VHL tend to present with neurological symptoms and signs at a younger age than those with sporadic disease. The syndrome is characterized by the development of multiple visceral and central nervous system (CNS) lesions. Tumors of the CNS include HBs, which affect 48-80% of patients with predisposing sites in the cerebellum (44-73%), brainstem (10-25%), spinal cord (13-50%), and retina (25-60%), as well as endolymphatic sac tumors, which affect 10-15% of patients (17-19). The visceral lesions include renal cell carcinoma, renal cyst, pheochromocytoma, pancreatic cyst, and pancreatic neuroendocrine tumor. Cystadenomas of the epididymis and broad ligament may also be present.
Disseminated HB is a distinct but very rare type of HB recurrence. Only 12 cases of disseminated HB have been reported previously (10-16). Mohan et al. (11) first reported two cases of disseminated HB that developed several years after complete excision. Bakshi et al. (10) reported a case of diffuse spinal leptomeningeal enhancement on MRI associated with multiple HBs and termed this condition hemangioblastomatosis. All 12 previously reported cases of disseminated HB arose in patients who had solid cerebellar HB as a primary lesion, and 9 of the patients had no association with VHL disease (10-16). All of the patients underwent surgical treatment of the primary lesion, and hemangiomatosis developed after variable intervals ranging from 6 months to 22 yr. In reported cases, biopsy specimens from the primary and secondary lesions were similar in histopathology to that in our case. No mitoses or signs of cellular anaplasia were seen. Tumor infiltration to leptomeninges was identified in five cases by MRI demonstrating diffuse leptomeningeal enhancement on the surface of the brainstem and spinal cord (10, 12, 14-16). The outcomes after recurrence were very poor, and most patients died within 3 yr. The most common cause of death was respiratory failure due to pontomedullary or cervical cord compression. The currently available treatment did not have a significant effect on the progression of the disease. Eight patients with disseminated HB received from 13 to 56 Gy of irradiation to the posterior fossa or spinal cord; however, long-term tumor control was not achieved (11-16). Advanced radiation techniques, such as radiosurgery, may be more effective than conventional fractionated radiotherapy, but it is difficult to perform high-dose radiation therapy or radiosurgery for numerous lesions scattered throughout the entire neuraxis. The use of interferon-α, thalidomide or a vascular endothelial growth factor receptor antagonist to inhibit angiogenesis (20, 21) did not result in remarkable tumor response.
Though VHL gene mutation was not detected in our case, the VHL gene may be mutated in sporadic cases of HB of the CNS (7). In a molecular genetic analysis of four cases of hemangioblastomatosis without VHL, Weil et al. (14) found no evidence of germline alterations in the VHL gene, but the somatic deletion of one copy of the VHL gene. These findings implicate a monoclonal origin of multiple, separate deposits of tumors in patients with sporadic HB. The results of comparative genomic hybridization studies suggest that other genes, in addition to the VHL gene, may be involved in the dissemination of HBs (15).
Because no case of de novo development of disseminated HB without previous surgery has been reported, it is strongly suggested that the spillage and spread of tumor cells through the CSF space may be an origin of hemangioblastomatosis in patients with a genetic predisposition to the condition. All previously reported cases showed multiple mass lesions exclusively in the infratentorial area and spinal cord except only one case (13). Our case is the second case uniquely presenting with additional lesions in supratentorial space. Paucity of supratentorial lesions may reflect CSF flow and effect of gravity supporting the hypothesis of tumor spread through CSF pathway. Preventive measures to reduce tumor cell spillage should be considered during operation, and further study to determine the biological risk factors of disseminated recurrence is needed.
Figures and Tables
Fig. 1
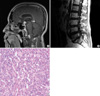
Initial magnetic resonance images, pathology finding and postoperative MR images in first diagnosis. (A, B) Initial T1-weighted magnetic resonance images obtained after gadolinium infusion. Sagittal and axial images of the brain showing a well-enhanced cerebellar mass but no mass in the suprasellar or cervicovertebral junction. (C) Pathology microphotograph stained with hematoxylin and eosin (×400) showing large vacuolated stromal cells that have larger, hyperchromatic nuclei and an eosinophilic foamy cytoplasm. It is characterized by the accumulation of lipid droplets within stromal cells and a rich capillary network. (D) T1-weighted magnetic resonance images taken 1 yr after surgery. There was no evidence of residual or recurrent disease on MR images.
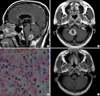
Fig. 2
Brain and lumbar magnetic resonance images and the pathology finding in disseminated recurrence. (A) T1-weighted magnetic resonance images obtained after gadolinium infusion. Sagittal view of the brain showing an enhancing mass in the suprasellar area with prominent leptomeningeal enhancement and another enhancing mass ventral to the upper spinal cord. (B) Sagittal view of the lumbar spine showing multiple enhancing nodules within the thoracolumbar spinal canal. (C) Histopathological photograph obtained from the mass in the cauda equina, which was stained with hematoxylin and eosin (×200). The architecture of tissue cells and morphology of the cells are identical with that of the cerebellar tumor shown in Fig. 1A.
References
1. Choyke PL, Filling-Katz MR, Shawker TH, Gorin MB, Travis WD, Chang R, Seizinger BR, Dwyer AJ, Linehan WM. von Hippel-Lindau disease: radiologic screening for visceral manifestations. Radiology. 1990. 174:815–820.

2. Choyke PL, Glenn GM, Walther MM, Patronas NJ, Linehan WM, Zbar B. von Hippel-Lindau disease: genetic, clinical, and imaging features. Radiology. 1995. 194:629–642.

3. Eisenhofer G, Lenders JW, Linehan WM, Walther MM, Goldstein DS, Keiser HR. Plasma normetanephrine and metanephrine for detecting pheochromocytoma in von Hippel-Lindau disease and multiple endocrine neoplasia type 2. N Engl J Med. 1999. 340:1872–1879.

4. Glasker S, Bender BU, Apel TW, van Velthoven V, Mulligan LM, Zentner J, Neumann HP. Reconsideration of biallelic inactivation of the VHL tumour suppressor gene in hemangioblastomas of the central nervous system. J Neurol Neurosurg Psychiatry. 2001. 70:644–648.

5. Neumann HP, Eggert HR, Weigel K, Friedburg H, Wiestler OD, Schollmeyer P. Hemangioblastomas of the central nervous system. A 10-year study with special reference to von Hippel-Lindau syndrome. J Neurosurg. 1989. 70:24–30.
6. Singounas EG. Haemangioblastomas of the central nervous system. Acta Neurochir (Wien). 1978. 44:107–113.

7. Lee JY, Dong SM, Park WS, Yoo NJ, Kim CS, Jang JJ, Chi JG, Zbar B, Lubensky IA, Linehan WM, Vortmeyer AO, Zhuang Z. Loss of heterozygosity and somatic mutations of the VHL tumor suppressor gene in sporadic cerebellar hemangioblastomas. Cancer Res. 1998. 58:504–508.
8. Richard S, David P, Marsot-Dupuch K, Giraud S, Beroud C, Resche F. Central nervous system hemangioblastomas, endolymphatic sac tumors, and von Hippel-Lindau disease. Neurosurg Rev. 2000. 23:1–22.

9. Niemela M, Lemeta S, Summanen P, Bohling T, Sainio M, Kere J, Poussa K, Sankila R, Haapasalo H, Kaariainen H, Pukkala E, Jaaskelainen J. Long-term prognosis of haemangioblastoma of the CNS: impact of von Hippel-Lindau disease. Acta Neurochir (Wien). 1999. 141:1147–1156.
10. Bakshi R, Mechtler LL, Patel MJ, Lindsay BD, Messinger S, Gibbons KJ. Spinal leptomeningeal hemangioblastomatosis in von Hippel-Lindau disease: magnetic resonance and pathological findings. J Neuroimaging. 1997. 7:242–244.

11. Mohan J, Brownell B, Oppenheimer DR. Malignant spread of haemangioblastoma: report on two cases. J Neurol Neurosurg Psychiatry. 1976. 39:515–525.

12. Reyns N, Assaker R, Louis E, Lejeune JP. Leptomeningeal hemangioblastomatosis in a case of von Hippel-Lindau disease: case report. Neurosurgery. 2003. 52:1212–1215.

13. Tohyama T, Kubo O, Kusano R, Miura N, Himuro H. A case of hemangioblastoma with subarachnoid dissemination. No Shinkei Geka. 1990. 18:83–88.
14. Weil RJ, Vortmeyer AO, Zhuang Z, Pack SD, Theodore N, Erickson RK, Oldfield EH. Clinical and molecular analysis of disseminated hemangioblastomatosis of the central nervous system in patients without von Hippel-Lindau disease. Report of four cases. J Neurosurg. 2002. 96:775–787.
15. Kato M, Ohe N, Okumura A, Shinoda J, Nomura A, Shuin T, Sakai N. Hemangioblastomatosis of the central nervous system without von Hippel-Lindau disease: a case report. J Neurooncol. 2005. 72:267–270.

16. Hanse MC, Vincent A, van den Bent MJ. Hemangioblastomatosis in a patient with von Hippel-Lindau disease. J Neurooncol. 2007. 82:163–164.

17. Hammel PR, Vilgrain V, Terris B, Penfornis A, Sauvanet A, Correas JM, Chauveau D, Balian A, Beigelman C, O'Toole D, Bernades P, Ruszniewski P, Richard S. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d'Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000. 119:1087–1095.
18. Hough DM, Stephens DH, Johnson CD, Binkovitz LA. Pancreatic lesions in von Hippel-Lindau disease: prevalence, clinical significance, and CT findings. AJR Am J Roentgenol. 1994. 162:1091–1094.

19. Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, Oldfield EH. von Hippel-Lindau disease. Lancet. 2003. 361:2059–2067.

20. Girmens JF, Erginay A, Massin P, Scigalla P, Gaudric A, Richard S. Treatment of von Hippel-Lindau retinal hemangioblastoma by the vascular endothelial growth factor receptor inhibitor SU5416 is more effective for associated macular edema than for hemangioblastomas. Am J Ophthalmol. 2003. 136:194–196.

21. Niemela M, Maenpaa H, Salven P, Summanen P, Poussa K, Laatikainen L, Jaaskelainen J, Joensuu H. Interferon alpha-2a therapy in 18 hemangioblastomas. Clin Cancer Res. 2001. 7:510–516.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download