Abstract
Dystrophic calcification can be defined as a calcification that occurs in degenerated or necrotic tissue. It is associated with multiple clinical conditions, such as collagen vascular diseases. It involves the deposition of calcium in soft tissues despite no generalized disturbance in the calcium or phosphorus metabolism, and this is often seen at sites of previous inflammation or damage. Potassium-titanyl phosphate (KTP) laser vaporization of the prostate is safe and relatively bloodless procedure that results in a shorter catheterization, immediate symptomatic improvement, and less severe postoperative irritative symptoms. However, longer follow-up studies or reports about complications are lacking. Here in we report a case of dystrophic calcification and stone formation on the entire bladder neck after performing KTP laser vaporization of benign prostate hyperplasia. That was treated by lithotripsy and transurethral resection.
Potassium-titanyl phosphate (KTP) laser vaporization of the prostate for treating symptomatic benign prostatic hypertrophy has recently proven to be efficacious with minimal patient morbidity. KTP laser vaporization can create an immediate cavity almost bloodlessly, with the added benefit of little-to-no learning curve and the prospect of successful, same-day, catheter-free discharge. However, longer follow-up studies or reports about possible complications are lacking.
Dystrophic calcification results from the deposition of calcium in soft tissues despite that there is no generalized disturbance in the calcium or phosphorus metabolism; this malady is often seen at sites of previous abnormalities or damage. The entity of dystrophic calcification is well described in the rheumatological and dermatological literature, and especially in the setting of dermatomyositis and scleroderma (1). However, reports of calcification in the genitourinary tract are not common. Here in we report an unusual case of dystrophic calcification of the bladder neck after performing KTP laser vaporization of the prostate.
A 78-yr-old man was presented with 1-yr history of dysuria, frequency and urgency. He had received KTP laser vaporization of the prostate at another hospital 16 months ago. According to past medical history, he had required catheterization for treating urinary retention during his postoperative care. Postoperative care had lasted 7 days, which was longer than usual, and then he had been discharged. Dysuria accompanied by frequency and urgency developed 4 months after the KTP laser vaporization.
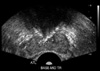
The laboratory results showed a plasma calcium level of 9.3 mg/dL and a plasma phosphorus level of 4.7 mg/dL, which were both within the normal ranges. The urinary sediment contained 10-29 white blood cells/high power field with 5-9 red blood cells/high power field, and bacterial culture of the urine was sterile. Transrectal ultrasonography of the prostate showed prostate volume of 9 mL and there was diffuse calcification around the internal urethral sphincter (Fig. 1). The cystoscopic findings revealed stones located in the bladder neck, and these were lodged into the previous surgical site. The stones were very tightly lodged into the mucosa of the bladder neck.
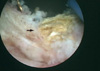
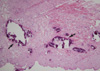
Cystoscopic examination was performed again under general anesthesia. The entire bladder neck was encircled by stones and even in the mucosa of the bladder neck. The stones were so tightly lodged into the mucosa of the bladder neck that they couldn't be disintegrated by performing lithotripsy in situ. Therefore, transurethral resection was performed on the mucosal-lodged stones and the calcified tissues (Fig. 2). The stones were bright yellowish (Fig. 3); they were determined to be calcium with phosphate on analysis. Hematoxylin and eosin staining of the surgical specimen showed extensive basophilic staining that was suggestive of calcification within the fibrotic tissues (Fig. 4).
Foley catheter was removed on the postoperative 4th day and uroflowmetry showed the peak/mean velocity to be 15/11 mL/sec and the self/residual urine volume was 210/30 mL. The bladder irritative symptoms completely disappeared by the postoperative 4 months follow-up.
Dystrophic calcification is associated with multiple clinical conditions. It often occurs in conjunction with such collagen vascular diseases as dermatomyositis, scleroderma and systemic lupus erythematosis (1). Various forms of trauma have also been implicated in dystrophic calcification. There have been reported cases of calcium deposition secondary to burn wounds, repeated trauma, needle sticks in the heels of neonates, thumb sucking by children, radiation and surgery (2-4). Although rarely seen in the genitourinary tract, it has been associated with schistosomiasis (5), renal and ureteral tumors (6), renal parenchymal disease (7), Churg-Strauss vasculitis (8), and polyarteritis nodosa (9). However, there have been no reports of dystrophic calcification and stones formation on the bladder neck associated with previous KTP laser vaporization of the prostate.
All mammalian extracellular fluids are supersaturated with calcium phosphate, but inhibitors such as matrix gla protein, oseoprotegrin and osteopotin prevent crystal deposition under normal physiological conditions (10, 11). Tissue injury promotes calcification in two ways. Damaged cell membranes leak calcium ions into cells, and these ions are subsequently concentrated by mitochondria to levels that are high enough to form crystals (2, 4). It has also been suggested that necrosis creates an acidic environment that lacks calcification inhibitors (12). Hydroxyapatite crystals are formed first within the protective microenvironment of the membrane microspace.
In rare cases, dystrophic calcification has been known to resolve spontaneously (1). Medical therapy, including administering etidronate disodium, sodium warfarin, diltiazem, aluminium hydroxide and intralesional corticosteroids, is the first-line treatment (1). The indications for surgery are the presence of painful masses, recurrent infection, ulceration, local functional impairment and cosmetic motivation (13). In the present case, the patient underwent surgery because
the calcific lesion caused the irritable bladder symptoms.
KTP laser vaporization of the prostate seems to combine the tissue-debulking properties of transurethral resection of prostate (TURP) with the well-known hemostatic properties of other laser treatments. It is a safe and relatively bloodless procedure that results in a shorter duration of catheterization, immediate symptomatic improvement and less severe postoperative irritative symptoms. However, concerning the efficacy and safety of this system, the majority of the studies about this deal with the short term results, which were excellent, but longer follow-up studies or reports about complications have been lacking. Bachman et al. conducted animal studies using ex-vivo, blood-perfused porcine kidneys, and they compared the hemostatic efficiency of KTP laser vaporization with TURP-like tissue resection. The authors showed the zone of coagulation necrosis was larger in the KTP group compared with the conventional TURP group (0.9 vs. 0.6 mm, respectively, P<0.01) and ablation of 16 cm3 of tissue was accomplished much faster in the TURP group (20 vs. 100 sec, respectively, P<0.0001) (14).
In this case, the patient had no systemic imbalances of calcium and phosphate metabolism and hadn't received any procedures or surgeries that used calcium solution. So, the development of the bladder neck calcification was likely to be due to dystrophic calcification that was caused by the previous operation. Additionally, the larger coagulation zone and longer operation time, compared to TURP, might have caused relatively more severe injury of the bladder neck and thus promoting dystrophic calcifications.
Figures and Tables
Fig. 1
The transrectal ultrasonography showed diffused calcification around the internal urethral sphincter.
References
1. Tristano AG, Villarroel JL, Rodriguez MA, Millan A. Calcinosis cutis universalis in a patient with systemic lupus erythematosus. Clin Rheumatol. 2006. 25:70–74.

2. Figueiredo GC, Figueiredo EC. Dystrophic calcinosis in a child with a thumb sucking habit: case report. Rev Hosp Clin Fac Med Sao Paulo. 2000. 55:177–180.

3. Lewis VJ, Holt PJ. Subcutaneous calcification following high-dose radiotherapy. Br J Dermatol. 2004. 150:1049–1050.

5. Merchant SA, Amonkar PP. Genitourinary bilharziasis: a review. Ind J Radiol Imag. 2002. 12:239–244.
6. Dyer RB, Chen MY, Zagoria RJ. Abnormal calcifications in the urinary tract. Radiographics. 1998. 18:1405–1424.

7. Tsujimura A, Imazu T, Nishimura K, Sugao H, Oka T, Takaha M, Takeda M, Kurata A. Ureteropelvic junction obstruction with renal pelvic calcification: a case report. J Urol. 1993. 150:1889–1890.

8. Symes A, Kalsi V, Rickards D, Allen C, Choong S, Philp T. Dystrophic ureteral calcification associated with Churg-Strauss vasculitis. Urology. 2004. 64:1231.

9. Gargollo PC, Barnewolt CE, Diamond DA. Calcified ureteral stricture in a child with polyarteritis nodosa. J Urol. 2004. 171:1254–1255.

10. Giachelli CM. Ectopic calcification: gathering hard facts about soft tissue mineralization. Am J Pathol. 1999. 154:671–675.
11. Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002. 161:2035–2046.

12. Verfaillie S, De Smet L, Leemans A, Van Damme B, Fabry G. Acute carpal tunnel syndrome caused by hydroxyapatite crystals: a case report. J hand Surg Am. 1996. 21:360–362.

13. Lipskeir E, Weizenbluth M. Calcinosis circumscripta: indications for surgery. Bull Hosp Jt Dis Orthop Inst. 1989. 49:75–84.
14. Reich O, Bachmann A, Schneede P, Zaak D, Sulser T, Hofstetter A. Experimental comparison of high power (80 W) potassium titanyl phosphate laser vaporization and transurethral resection of prostate. J Urol. 2004. 171:2502–2504.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download