Abstract
High-dose chemotherapy and autologous stem cell rescue (HDCT/ASCR) was applied to improve the prognosis of patients with high-risk stage 3 neuroblastoma. From January 1997 to December 2006, 28 patients were newly diagnosed as stage 3 neuroblastoma. Nine of 11 patients with N-myc amplification and 5 of 17 patients without N-myc amplification (poor response in 2 patients, persistent residual tumor in 2 and relapse in 1) underwent single or tandem HDCT/ASCR. Patients without high-risk features received conventional treatment modalities only. While 8 of 9 patients underwent single HDCT/ASCR and the remaining one patient underwent tandem HDCT/ASCR during the early study period, all 5 patients underwent tandem HDCT/ASCR during the late period. Toxicities associated with HDCT/ASCR were tolerable and there was no treatment-related mortality. While the tumor relapsed in two of eight patients in single HDCT/ASCR group, all six patients in tandem HDCT/ASCR group remained relapse free. The 5-yr event-free survival (EFS) from diagnosis, in patients with N-myc amplification, was 71.6±14.0%. In addition, 12 of 14 patients who underwent HDCT/ASCR remained event free resulting in an 85.1±9.7% 5-yr EFS after the first HDCT/ASCR. The present study demonstrates that HDCT/ASCR may improve the survival of patients with high-risk stage 3 neuroblastoma.
The prognosis of stage 3 neuroblastoma is generally good (1, 2). However, the prognosis of stage 3 neuroblastoma with unfavorable biologic factors, such as, N-myc amplification, is poor with conventional chemoradiotherapy (1, 3). In addition, the prognosis of stage 3 neuroblastoma with a poor response to conventional treatment (4-6) or with relapse during conventional treatment is also poor even when the tumor did not have unfavorable biologic factors.
A strategy using high-dose chemotherapy and autologous stem cell rescue (HDCT/ASCR) has been explored to improve the prognosis of patients with high-risk neuroblastoma (7-11). This strategy is based on the hypothesis that a dose escalation might improve the survival of children with high-risk neuroblastoma. The results of randomized trials, comparing HDCT/ASCR with chemotherapy alone, showed a better event-free survival (EFS) in the HDCT/ASCR arm than in the continuous chemotherapy arm (8, 11). Recently, investigators have examined the efficacy of double or triple tandem HDCT/ASCR to further improve the outcome of high-risk neuroblastoma patients. Sung et al. (12) and George et al. (13) carried out a single arm trial of tandem transplantation as consolidation therapy, and reported improved long-term survival (5-yr progression-free survival 62% and 47%, respectively) with acceptable toxicity. Kletzel et al. also conducted a single arm trial of triple tandem transplantation and reported improved survival (3-yr EFS 57%) (14). They demonstrated that further dose escalation using sequential HDCT/ASCR might result in further improvements in the survival of patients with high-risk neuroblastoma.
However, a majority of patients in their studies had stage 4 tumors and only a small proportion of patients had stage 3 high-risk tumors. Therefore, the efficacy of HDCT/ASCR for stage 3 high-risk patients was not shown in their reports (7-14). The number of studies evaluating the efficacy of HDCT/ASCR only in patients with high-risk stage 3 neuroblastoma is limited. In this context, the present study examined the efficacy of HDCT/ASCR in order to determine if a dose intensification strategy might improve the EFS in patients with high-risk stage 3 neuroblastoma.
From January 1997 to December 2006, 28 consecutive patients were newly diagnosed as stage 3 neuroblastoma at the Samsung Medical Center. A diagnosis of neuroblastoma was made based on either histological examination of the tumor specimens or bone marrow infiltrated with neuroblastoma cells and elevated urine catecholamine levels. The N-myc copy number was determined using Southern blot analysis or quantitative reverse transcriptase-polymerase chain reaction (RT-PCR). The patients were staged according to the criteria reported by Brodeur et al. (15) and the extent of the disease was evaluated using computerized tomography, a technetium-99 (99Tc) bone scan, bilateral bone marrow aspirates and biopsy specimens, and an iodine-131 or 123-metaiodobenzylguanidine scan. Measurement of serum lactate dehydrogenase, ferritin and neuron-specific enolase was part of the routine evaluation at diagnosis.
Fig. 1 summarizes the treatment. Most patients received 5-6 cycles of chemotherapy before definitive surgery, except for those patients that had a surgical resection before the administration of chemotherapy. If it was difficult to debulk the tumor, an additional 1-2 cycles of chemotherapy were given prior to the definitive surgery.
For patients with N-myc amplified tumors, patients with a very poor response to chemotherapy or patients with persistent gross residual tumor after surgery, the peripheral blood stem cells (PBSCs) were collected during the next chemotherapy cycle after surgery. After collecting the PBSCs, 0-2 additional cycles of chemotherapy were given prior to the HDCT/ASCR. For patients without N-myc amplification who responded well, continuous chemotherapy was provided after surgery (total 12 cycles).
Table 1 lists the chemotherapy regimens used in the present study. During the early period of the study (diagnosis by December 2003), the cisplatin, etoposide, doxorubicin and cyclophosphosphamide (CEDC) regimen was used as the primary treatment. In the late period (diagnosis from January 2004), alternating CEDC and ifosfamide, carboplatin and etoposide (ICE) regimens were used. While all the patients in the late study period received 9 cycles of chemotherapy prior to the HDCT/ASCR, 7 of 9 patients in the early study period received 6 or 7 cycles of chemotherapy. Each chemotherapy cycle was scheduled to be 28 days apart but some delays were permitted in order to allow the absolute neutrophil count (ANC) and platelet count to recover to 1,000/µL and 100,000/µL, respectively. The patients received 5-10 µg/kg of subcutaneous human granulocyte-colony stimulating factor (G-CSF) daily if the ANC fell below 500/µL after chemotherapy.
The patients received 5-10 µg/kg of subcutaneous G-CSF daily if the ANC fell below 500/µL after chemotherapy, and G-CSF was continued until the completion of leukapheresis. Leukapheresis was started when the white blood cell count exceeded 1,000/µL with a monocytosis after the nadir. The aim was to collect a minimum of 2×106 CD34+ cells/kg (hopefully >5×106 cells/kg) in order to rescue the probable tandem HDCT with the PBSCs collected during a single leukapheresis round.
Table 1 lists the HDCT regimens used in the present study. While only one patient who was not in complete response (CR), even after the first HDCT/ASCR, underwent a second HDCT/ASCR in the early period of this study, all the patients in the late period underwent tandem HDCT/ASCR. The HDCT regimens employed for the first HDCT were carboplatin, etoposide and melphalan (CEM) in the early period and CEC in the late period. The regimens employed for the second HDCT were carboplatin, thiotepa, and melphalan (CTM) in the early period and TM in the late period. Total body irradiation (TBI) was not included in the HDCT regimen. The second HDCT/ASCR was given only if the platelet count exceeded 50,000/µL after the first HDCT/ASCR, without a transfusion, and if there was no evidence of significant organ dysfunction. Approximately half of the collected PBSCs were infused for marrow rescue at each HDCT session. Samsung Medical Center Institutional Review Board approved the protocols used for the HDCT/ASCR, and written informed consent was obtained from the parents of each patient.
In the early period of this study, local radiotherapy (15 Gy/1.5 Gy in the case of small residual tumor and 21.6 or 30.6 Gy/1.8 Gy in the case of bulky residual tumor) was given to the primary site only if gross residual tumor remained after surgery. However, in the late period, local radiotherapy (15 Gy/1.5 Gy in the case of complete resection of the primary tumor and 21.6 Gy/1.8 Gy in the case of incomplete resection) was administered to all patients (4 weeks after the completion of conventional chemotherapy or 6 weeks after the second HDCT/ASCR).
Thirteen-cis-retinoic acid (CRA) was used for 10 cycles (125 mg/m2/day for 14 days per every 4 weeks) to differentiate the possible minimal residual tumor cells. Differentiation therapy with CRA was initiated 60-90 days after the HDCT/ASCR or 30 days after the completion of conventional chemotherapy. Immunotherapy using interleukin-2 (IL-2) was administered (2×106 U/m2/day, subcutaneous injection, days 0-4, every 4 weeks) with or without preceding induction therapy (3×106 U/m2/day, continuous infusion, days 0-4 and 7-11) (16). IL-2 immunotherapy was initiated when the platelet count exceeded 50,000/µL, without a transfusion, after the HDCT/ASCR. The first dose of IL-2 was provided on the first day of the CRA treatment cycle and was continued until 1 yr after the HDCT/ASCR. Immunotherapy was not used in patients who received conventional chemotherapy alone.
The international response criteria were used to evaluate the treatment response (15). Briefly, a CR was defined as no identifiable tumor with normal catecholamine levels. A very good partial response (VGPR) was defined as a decrease in the primary tumor by 90-99% with normal catecholamine levels with or without any residual 99Tc bone changes. A partial response (PR) was defined as a reduction of the primary tumor and metastatic tumor by more than 50%. The toxicity was recorded according to the common toxicity criteria of the National Cancer Institute of the U.S.A.
The survival rate along with the standard error (SE) was estimated using the Kaplan-Meier method. An event was defined as the occurrence of a relapse, progression or treatment-related death. The differences in the survival rates between the two groups (single HDCT versus tandem HDCT, N-myc amplified versus not amplified) were compared using the log-rank test. The differences in the ANC and platelet recovery between the first and second HDCT/ASCR were analyzed using a paired t-test. P values <0.05 were considered significant.
Table 2 lists the clinical and biological characteristics of all 28 patients. The median age of the 28 patients (15 males and 13 females) at diagnosis was 23.5 months (range 1-95), and 19 patients (67.9%) were over 12 months of age at diagnosis. Eleven out of 28 tumors (39.3%) were N-myc amplified (≥3 copies) and 10 (35.7%) had unfavorable Shimada pathology. The primary site of the tumors was the abdomen in 23 patients, mediastinum in 4 patients and neck in 1 patient.
Fig. 1 shows the flow of patients from diagnosis through HDCT/ASCR. All except two patients with N-myc amplification (early death in 1 and refusal to receive HDCT in 1) underwent HDCT/ASCR. In addition, five of 17 patients without N-myc amplification (poor response to chemotherapy in 2 patients, persistent gross residual tumor in 2 patients and relapse during chemotherapy in 1 patient) underwent HDCT/ASCR. Therefore, overall, 14 patients underwent HDCT/ASCR. Eleven of 17 patients without N-myc amplification received conventional chemotherapy alone.
Table 3 lists the clinical and biological characteristics of 14 patients who underwent HDCT/ASCR. Overall, a median of 8 cycles (range 6-14) of chemotherapy was administered before the first HDCT/ASCR. Five out of 14 patients received local radiotherapy after surgery. The tumor status prior to the first HDCT was CR in 7, VGPR in 5 and PR in 2.
The PBSCs were collected during the sixth to eighth chemotherapy cycle. A median of 6.5×106 CD34+ cells/kg (range 2.1-17.9) were collected during a median of four leukapheresis events (range 2-11). Generally, the leukapheresis procedure was well tolerated and no patient developed significant toxicity except for thrombocytopenia requiring a transfusion after leukapheresis in all patients. During the late period of this study, quantitative RT-PCR for tyrosine hydroxylase mRNA was performed to detect possible tumor cell contamination in the PBSCs collected from five patients, and no mRNA transcript was detected (12).
Fourteen out of 28 patients underwent at least one HDCT/ASCR. The median age at the first HDCT/ASCR was 30.5 months (range 16-70). The tumor status after the first HDCT/ASCR was CR in 12 and VGPR in 2. One out of nine patients in the early period and all five in the late period underwent a second HDCT/ASCR. The median time from the first infusion of PBSCs to the initiation of the second HDCT was 82.5 days (range 66-88).
A median of 4.0×106 CD34+ cells/kg (range 0.6-13.0) were infused for the first HDCT/ASCR, and the median time required to reach an ANC of 500/µL and a platelet count of 20,000/µL, without transfusion for the previous 7 days, was 10 days (range 8-14) and 24 days (range 16-38), respectively. A median of 4.1×106 CD34+ cells/kg (range 1.5-7.3) were infused for the second HDCT/ASCR and the median time required to reach an ANC of 500/µL and a platelet count of 20,000/µL was 10 days (range 8-14) and 19.5 days (range 14-220), respectively. When the analysis was confined to only those patients who had received tandem transplantation, there were no significant differences in the ANC and platelet recovery between the first and second HDCT/ASCR.
Table 4 lists the grade 3 and 4 toxicities that developed during the first and second HDCT/ASCR. The median duration of high fever (body temperature ≥38.0℃) during the first and second HDCT/ASCR was 5 and 6.5 days, respectively. There were three microbiologically documented infections (2 in the first and 1 in the second HDCT/ASCR). The grade 3 and 4 toxicities that frequently developed in more than 1/3 of patients were vomiting, elevation of liver enzymes and hypokalemia. There was no toxic death during the first and second HDCT/ASCR. No secondary malignancy developed in this study.
Skin eruption, particularly on the face, was a common side effect of the CRA treatment. High fever, thrombocytopenia and local inflammation were frequent side effects of the IL-2 therapy. However, all patients tolerated the differentiation therapy and immunotherapy well and there was no grade 3 or 4 toxicity that required the discontinuation of treatment during the post-HDCT/ASCR therapy.
The tumor relapsed in three out of all 28 patients and there were two toxic deaths during the conventional chemotherapy. Therefore, 23 out of 28 patients remained event free with a median follow-up of 63 months (range 12-129) from diagnosis. The 5-yr EFS rate (±SE) after diagnosis for all 28 patients was 80.8±7.8% (Fig. 2A). The 5-yr EFS rate, for all 11 patients with N-myc amplification and the 17 patients without, was 71.6±14.0% and 87.8±8.1%, respectively (Fig. 2B).
The tumor relapsed in 2 out of 14 patients who underwent HDCT/ASCR and the remaining 12 patients remained event free with a median follow-up of 55 months (range 12-110) from the first HDCT/ASCR. The 5-yr EFS rate after the first HDCT/ASCR for all 14 patients who underwent single or tandem HDCT/ASCR, was 85.1±9.7% (Fig. 3A). While the tumor relapsed in two of eight patients who underwent single HDCT/ASCR, all six patients who underwent the tandem HDCT/ASCR remained event free (Fig. 3B).
Although the prognosis of stage 3 neuroblastoma is generally good (1, 2), the prognosis of stage 3 neuroblastoma with unfavorable biologic factors, especially N-myc amplification, is poor with conventional chemoradiotherapy (1, 3). In addition, the prognosis of stage 3 neuroblastoma with a poor response to conventional treatment (4-6) or with a relapse during conventional treatment is also poor even when the tumor did not have unfavorable biologic factors. In this context, HDCT/ASCR was given to patients with high-risk stage 3 neuroblastoma in the present study. As a result, the 5-yr EFS from diagnosis, in patients with N-myc amplified tumor, was 71.6±14.0%. In addition, 12 out of 14 patients who underwent HDCT/ASCR remained event free resulting in an 85.1±9.7% 5-yr EFS after the first HDCT/ASCR. Survival rate in the present study is higher than those reported in the previous trials in which conventional chemotherapy was given for the treatment of high-risk stage 3 neuroblastoma (1, 3). These findings suggest that HDCT/ASCR might improve the survival of patients with high-risk stage 3 neuroblastoma.
Generally, the prognosis of stage 3 neuroblastoma without N-myc amplification is good with conventional chemotherapy alone (1, 2). However, even in these patients, the prognosis is poor, if the tumor response to conventional chemotherapy is poor (4) or gross residual tumor remained after surgery (5, 6). In this context, HDCT/ASCR was given to two patients with a poor response to conventional chemotherapy and two patients with a gross residual mass after surgery as well as patients with N-myc amplified tumor. These four patients remained event free; the 5-yr EFS for patients without N-myc amplification was 87.8±8.1% in the present study. In the present study, HDCT/ASCR contributed to the excellent survival rate in patients without N-myc amplification in whom the tumor response to conventional chemotherapy was poor.
In our recent report, on patients over 1 yr of age with stage 4 neuroblastoma, the EFS in patients who received TBI was better than for patients who did not receive TBI (12). This suggested that a regimen with TBI was better than a regimen without, for the survival of patients, over 1 yr of age, with stage 4 neuroblastoma. However, TBI was not included in the HDCT regimen for high-risk stage 3 patients in the present study. Although the tumor relapsed in 2 of 14 patients who underwent HDCT/ASCR, the remaining 12 high-risk patients remained event free without TBI. TBI may increase the frequency and severity of short-term side effects as well as long-term side effects such as a secondary malignancy several years after treatment (17, 18). The fact that there was no toxicity-related death during the HDCT/ASCR, in the present study, might be attributed to the absence of TBI in our HDCT regimen. Taken together, these findings suggest that our regimen without TBI might be safe and effective for patients with high-risk stage 3 neuroblastoma.
Although the strategy with the HDCT/ASCR improved the survival of patients with high-risk neuroblastoma, the survival rate after a single HDCT/ASCR has been unsatisfactory (7-11). Our recent report, which examined the efficacy of tandem HDCT/ASCR to further improve the treatment outcome of stage 4 neuroblastoma patients over 1 yr of age, showed a better long-term EFS compared to studies reporting on a single HDCT/ASCR strategy (12). The strategy using a tandem HDCT/ASCR was based on the hypothesis that further dose escalation might result in further improvements in the EFS of high-risk neuroblastoma patients. In the present study, the tandem HDCT/ASCR was given only if a CR was not achieved, even after the first HDCT/ASCR, in the early period of the study. However, all patients underwent tandem HDCT/ASCR during the late study period. As a result, while the tumor relapsed in two of eight patients in the single HDCT group, all 6 patients in the tandem HDCT group remained relapse free. These findings suggest that the tandem HDCT strategy may be better than the single HDCT strategy for improved survival even in patients with high-risk stage 3 neuroblastoma, although it was not statistically significant in the present study. Further study will be needed to evaluate whether tandem HDCT/ASCR is better than single HDCT/ASCR in the treatment of high-risk stage 3 neuroblastoma. In six patients who underwent tandem HDCT/ASCR, the short-term toxicities were acceptable during the second HDCT/ASCR. There was no life-threatening toxicity during the second HDCT/ASCR. However, long-term follow-up is needed to evaluate the long-term toxicities of the tandem HDCT/ASCR.
According to previous reports, patients treated for longer periods, prior to HDCT/ASCR, had better long-term EFS than those patients treated for shorter periods (9, 12). While all the patients in the late study period received 9 cycles of chemotherapy prior to the HDCT/ASCR, most of the patients in the early study period received 6 or 7 cycles of chemotherapy. The two patients in whom the tumor relapsed after the HDCT/ASCR received 6 cycles of chemotherapy prior to HDCT/ASCR, although they were in the CR or VGCR prior to the HDCT/ASCR. Similarly, the tumor relapsed in two of six patients who received 6 cycles of chemotherapy prior to the HDCT, although the tumor status prior to the first HDCT was CR in three patients and VGPR in three, respectively. However, it is unclear whether the short duration of conventional chemotherapy was related to the high relapse rate after HDCT/ASCR in the present study, because all but one patient in the single HDCT/ASCR group received 6 or 7 cycles of chemotherapy prior to the first HDCT/ASCR while all patients in the tandem HDCT/ASCR group received 9 cycles of chemotherapy.
Immunotherapy with IL-2 along with CRA was provided in an attempt to control the minimal residual disease after the HDCT/ASCR. These two drugs were used simultaneously because their major toxicities do not overlap and their different mechanisms of action could synergistically eradicate any minimal residual disease. While the efficacy of CRA has been demonstrated (19-21), there have been few studies that have evaluated the efficacy of IL-2 therapy (16). Therefore, it is unclear if IL-2 therapy contributed to the improved survival in the present study. However, there was no life-threatening toxicity and an increase in the number of natural killer cells was observed after IL-2 therapy (22). More study will be needed to evaluate the efficacy of the IL-2 treatment after HDCT/ASCR.
The present study demonstrates that HDCT/ASCR, especially tandem HDCT/ASCR, may improve the prognosis of patients with high-risk stage 3 neuroblastoma. However, throughout this study, multiple modifications were made in the treatment plan, which resulted in significant variability over time between the patients. This variability may create doubt as to whether tandem HDCT/ASCR actually resulted in the improved outcomes. For example, a longer pre-HDCT treatment in the tandem HDCT group compared with the single HDCT group might have had an impact on the EFS in the present study. Further study will be needed to evaluate the efficacy of tandem HDCT/ASCR in the treatment of high-risk stage 3 neuroblastoma.
Figures and Tables
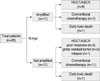 | Fig. 1Flow of the patients from diagnosis through HDCT/ASCR. All except two patients with N-myc amplification (early death in 1 and refusal to receive HDCT in 1) underwent HDCT/ASCR. In addition, 5 of 17 patients without N-myc amplification (poor response to chemotherapy in 2 patients, persistent gross residual tumor in 2 patients and relapse during chemotherapy in 1 patient) underwent HDCT/ASCR. Therefore, overall 14 patients underwent HDCT/ASCR. Eleven of 17 patients without N-myc amplification received conventional chemotherapy alone. |
 | Fig. 2Results of Kaplan-Meier analysis for the EFS in all 28 patients. The tumor relapsed in 3 out of 28 patients and there were 2 toxic deaths during conventional chemotherapy. Therefore, 23 out of 28 patients remained event free with a median follow-up of 63 months (range 12-129) from diagnosis. (A) The 5-yr EFS rate (±SE) after diagnosis for all 28 patients was 80.8±7.8%. (B) The 5-yr EFS rate for all 11 patients with N-myc amplification and 17 patients without was 71.6±14.0% and 87.8±8.1%, respectively. |
 | Fig. 3Results of Kaplan-Meier analysis for the EFS of 14 patients who underwent HDCT/ASCR. The tumor relapsed in 2 out of 14 patients and the remaining 12 patients remained event free with a median follow-up of 55 months (range 12-110) from the first HDCT/ASCR. (A) The 5-yr EFS rate after the first HDCT/ASCR for all 14 patients was 85.1±9.7%. (B) While tumor relapsed in 2 of 8 patients in single HDCT/ASCR group, all 6 patients in tandem HDCT/ASCR group remained event free. |
ACKNOWLEDGMENTS
We thank the residents and nurses who cared for the patients, and without whom this study would not have been possible.
References
1. Matthay KK, Perez C, Seeger RC, Brodeur GM, Shimada H, Atkinson JB, Black CT, Gerbing R, Haase GM, Stram DO, Swift P, Lukens JN. Successful treatment of stage III neuroblastoma based on prospective biologic staging: a Children's Cancer Group study. J Clin Oncol. 1998. 16:1256–1264.

2. Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005. 17:7–13.

3. Rubie H, Hartmann O, Michon J, Frappaz D, Coze C, Chastagner P, Baranzelli MC, Plantaz D, Avet-Loiseau H, Benard J, Delattre O, Favrot M, Peyroulet MC, Thyss A, Perel Y, Bergeron C, Courbon-Collet B, Vannier JP, Lemerle J, Sommelet D. N-Myc gene amplification is a major prognostic factor in localized neuroblastoma: results of the French NBL 90 study. Neuroblastoma Study Group of the Société Francaise d'Oncologie Pédiatrique. J Clin Oncol. 1997. 15:1171–1182.

4. Castel V, Garcia-Miguel P, Canete A, Melero C, Navajas A, Ruiz-Jimenez JI, Navarro S, Badal MD. Prospective evaluation of the International Neuroblastoma Staging System (INSS) and the International Neuroblastoma Response Criteria (INRC) in a multicentre setting. Eur J Cancer. 1999. 35:606–611.

5. La Quaglia MP, Kushner BH, Su W, Heller G, Kramer K, Abramson S, Rosen N, Wolden S, Cheung NK. The impact of gross total resection on local control and survival in high-risk neuroblastoma. J Pediatr Surg. 2004. 39:412–417.

6. von Schweinitz D, Hero B, Berthold F. The impact of surgical radicality on outcome in childhood neuroblastoma. Eur J Pediatr Surg. 2002. 12:402–409.

7. Stram DO, Matthay KK, O'Leary M, Reynolds CP, Haase GM, Atkinson JB, Brodeur GM, Seeger RC. Consolidation chemoradiotherapy and autologous bone marrow transplantation versus continued chemotherapy for metastatic neuroblastoma: a report of two concurrent Children's Cancer Group studies. J Clin Oncol. 1996. 14:2417–2426.

8. Matthay KK, Villablanca JG, Seeger RC, Stram DO, Harris RE, Ramsay NK, Swift P, Shimada H, Black CT, Brodeur GM, Gerbing RB, Reynolds CP. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999. 341:1165–1173.
9. Ladenstein R, Philip T, Lasset C, Hartmann O, Garaventa A, Pinkerton R, Michon J, Prichard J, Klingebiel T, Kremens B, Pearson A, Coze C, Paolucci P, Frappaz D, Gadner H, Chauvin F. Multivariate analysis of risk factors in stage 4 neuroblastoma patients over the age of one year treated with megatherapy and stem-cell transplantation: a report from the European Bone Marrow Transplantation Solid Tumor Registry. J Clin Oncol. 1998. 16:953–965.

10. Cohn SL, Moss TJ, Hoover M, Katzenstein HM, Haut PR, Morgan ER, Green AA, Kletzel M. Treatment of poor-risk neuroblastoma patients with high-dose chemotherapy and autologous peripheral stem cell rescue. Bone Marrow Transplant. 1997. 20:543–551.

11. Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, Klingebiel T, Kremens B, Schilling FH, Schrappe M, Simon T, Hero B. Myeloablative megatherapy with autologous stem-cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high-risk neuroblastoma: a randomized controlled trial. Lancet Oncol. 2005. 6:649–658.
12. Sung KW, Lee SH, Yoo KH, Jung HL, Cho EJ, Koo HH, Lee SK, Kim J, Lim DH, Suh YL, Kim DW. Tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma. Bone Marrow Transplant. 2007. 40:37–45.

13. George RE, Li S, Medeiros-Nancarrow C, Neuberg D, Marcus K, Shamberger RC, Pulsipher M, Grupp SA, Diller L. High-risk neuroblastoma treated with tandem autologous peripheral-blood stem cell-supported transplantation: long-term survival update. J Clin Oncol. 2006. 24:2891–2896.

14. Kletzel M, Katzenstein HM, Haut PR, Yu AL, Morgan E, Reynolds M, Geissler G, Marymount MH, Liu D, Kalapurakal JA, Shore RM, Bardo DM, Schmoldt J, Rademaker AW, Cohn SL. Treatment of high-risk neuroblastoma with triple-tandem high-dose therapy and stem-cell rescue: results of the Chicago Pilot II Study. J Clin Oncol. 2002. 20:2284–2292.

15. Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, Evans AE, Favrot M, Hedborg F. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993. 11:1466–1477.

16. Pession A, Prete A, Locatelli F, Pierinelli S, Pession AL, Maccario R, Magrini E, De Bernardi B, Paolucci P, Paolucci G. Immunotherapy with low-dose recombinant interleukin 2 after high-dose chemotherapy and autologous stem cell transplantation in neuroblastoma. Br J Cancer. 1998. 78:528–533.

17. Flandin I, Hartmann O, Michon J, Pinkerton R, Coze C, Stephan JL, Fourquet B, Valteau-Couanet D, Bergeron C, Philip T, Carrie C. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society of Pediatric oncology. Int J Radiat Oncol Biol Phys. 2006. 64:1424–1431.

18. Meacham LR, Gurney JG, Mertens AC, Ness KK, Sklar CA, Robison LL, Oeffinger KC. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005. 103:1730–1739.
19. Finklestein JZ, Krailo MD, Lenarsky C, Ladisch S, Blair GK, Reynolds CP, Sitarz AL, Hammond GD. 13-cis-retinoic acid (NSC 122758) in the treatment of children with metastatic neuroblastoma unresponsive to conventional chemotherapy: report from the Childrens Cancer Study Group. Med Pediatr Oncol. 1992. 20:307–311.

20. Kohler JA, Imeson J, Ellershaw C, Lie SO. A randomized trial of 13-Cis retinoic acid in children with advanced neuroblastoma after high-dose therapy. Br J Cancer. 2000. 83:1124–1127.

21. Matthay KK, Reynolds CP. Is there a role for retinoids to treat minimal residual disease in neuroblastoma? Br J Cancer. 2000. 83:1121–1123.

22. Shin MY, Ahn KM, Sung KW, Koo HH. Immunotherapy with interleukin-2 after autologous stem cell transplantation in children with high-risk solid tumor. Korean J Hematol Stem Cell Trans. 1999. 4:239–248.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download