Abstract
In the patients with brain metastasis (BM), it is impossible to determine who will benefit from surgery because of limited survival. In an attempt to identify optimal candidates for brain metastatectomy, we analyzed patients who survived for <3 months after craniotomy for a single BM lesion. Between January 1st, 1997 and July 31st, 2007, 83 patients with a single BM underwent craniotomy. Of these patients, 25 patients (30.1%) died within 3 months of craniotomy. The primary lesions were non-small call lung cancer in 15, colon cancer in 6, and breast cancer, renal cell carcinoma, ovarian cancer, or esophageal cancer in one apiece. Of the 25 patients, 19 (79%) were of tumor stage IV and had extra-cranial metastasis. Eleven (44%) of the 25 primary cancers had a well-controlled status. Twelve patients (48%) had a Karnofsky Performance Scale (KPS) score of <70, and 13 (52%) were of Recursive Partitioning Analysis (RPA) class 3. Primary cancer status, RPA class, and functional status were found to be critical factors for consideration when selecting surgical candidates. In addition, adjuvant therapy was found to have an important role on survival.
It has been estimated that up to 170,000 new cases of brain metastases (BM) occur each year in the Unites States (1, 2), and clinical studies have shown that between 20 and 30% of patients with non-small cell lung cancer (NSCLC) develop BM at some time during the course of their disease (3). In the largest autopsy series performed to date, Posner and Chernik examined the records of 2,375 cancer patients and found that central nervous system metastases occur in approximately 24% of patients with systemic cancers (4).
Some of these patients can be treated by craniotomy. Historically, some BM patients could be treated by craniotomy for palliation, which has been usually combined with brain irradiation. However, due to advances in surgical techniques, more lesions are now considered accessible, and surgical complications and post-surgical morbidity have been reduced. Moreover, modern imaging techniques enable smaller metastases to be located and defined, and allow operations to be undertaken earlier during BM development.
Actually, brain metastatectomy offers certain advantages over other therapeutic modalities (5). First, the excision of a metastasis can immediately eliminate the effects of increased intracranial pressure and the direct symptomatic effects on surrounding brain tissues. Second, surgery provides tissue for confirming a diagnosis of metastasis. Third, surgery may establish a local cure or complete remission if all tumor cells can be removed.
However, despite the above advantages of BM surgery, it has proven to offer limited benefits because of high recurrence rates and short survival after resection. Therefore, when potential BM candidates for resection are considered, it is not possible to determine precisely who will benefit from surgical resection and post-resection treatment, because of the limited survival associated with metastatic brain tumors. Although some guidelines for BM treatment, such as, 'Clinical Practice Guidelines in Oncology' by the National Comprehensive Cancer Network (NCCN) (6), have been issued, many controversies regarding treatment remain. Therefore, in an attempt to identify optimal candidates for brain metastatectomy, we retrospectively evaluated the clinical records of 25 patients who survived for less than 3 months after brain metastatectomy, and compared with all the patients who underwent brain metastatectomy during same period at the same institutes.
From January 1997 through July 2007, 83 patients with a single BM lesion underwent craniotomy for brain tumor resection at Masan Samsung Hospital and Dong-A University Medical Center. Before brain metastatectomy, we expected all 83 patients to survive for >12 weeks based on their preoperative medical conditions, but as many as 25 (30.1%) died within 3 months of craniotomy.
A retrospective analysis was performed on the clinical variables of the 25 cases that died within 3 months of craniotomy. These variables included patient age and gender, type of primary cancer, primary tumor stage, presence of extra-cranial metastasis, status of primary cancer, Karnofsky Performance Scale (KPS) score (7), and Recursive Partitioning Analysis (RPA) class.
According to the classification devised by Gaspar et al. (8), patients in RPA class 1 are characterized by an age of <65 yr, a KPS score of ≥70, the absence of extra-cranial metastases, and good control of systemic disease. RPA class 2 patients have a KPS score of ≥70, but may also have an age of ≥65 yr, or uncontrolled systemic disease or systemic metastases. Patients in RPA class 3 are those with a KPS score of <70.
The parameters in the 25 patients examined included metastasis timing, size and location of BM, and functional grade of brain lesion. Brain metastases diagnosed <60 days after primary lesion diagnosis were considered synchronous metastases, and those diagnosed ≥60 days of primary lesion diagnosis were considered metachronous metastases. Sizes of BM were defined as maximal orthogonal diameter in T1 weighted gadolinium (Gd) enhanced magnetic resonance (MR) images. Locations of BM were categorized as supratentorial or infratentorial. Each tumor was functionally graded by preoperative MRI in terms of location with respect to the eloquent brain according to the scheme developed at the M.D. Anderson Cancer Center (9). Functional grade I tumors were located in non-eloquent brain regions (e.g., frontal or temporal polar areas), grade II tumors near eloquent brain regions (e.g., near the motor or sensory cortex, calcarine fissure, speech center, dentate nucleus, brain stem, or corpus callosum) and grade III tumors were located in eloquent brain regions (e.g., motor and sensory cortex, visual center, speech center, internal capsule, basal ganglia, hypothalamus, thalamus, brain stem, or dentate nucleus).
Clinical indications included symptoms and signs of intracranial hypertension unresponsive to adequate medical therapy, (e.g., corticosteroid and mannitol), intractable seizures, reduced level of consciousness, progressive motor weakness, gait ataxia, or aphasia. Neuro-imaging indications included lesion enlargement, associated hemorrhage, and a mass effect due to edema unresponsive to maximal medication.
All the 25 patients had life expectancies of >12 weeks and had not undergoing investigational therapies during the preceding 3 weeks. Patients that had undergone prior irradiation and those with leptomeningeal tumor involvement were excluded.
The medical records, i.e., clinical history, operative and pathology reports, and radiographic studies, of the 25 patients were analyzed, and dates of death were confirmed for all patients that succumbed.
Survival times were calculated from the dates of BM surgery to death. In addition, dates of primary cancer diagnosis, dates of BM diagnosis, dates of brain metastatectomy, and dates of death were recorded, and times between these dates were calculated. Overall survival was defined as time from date of primary cancer diagnosis to death.
Post-surgical imaging studies were reviewed for evidence of central nervous system (CNS) progression. On Gd enhanced T1 weighted MR images, CNS progression was defined as the appearance of a new enhancing brain lesion including original BM resection site, or in the spinal cord or cerebrospinal fluid. In the patients that died, cause of death and date of death were recorded. Causes of death were categorized as CNS-related if a patient had stable systemic disease but succumbed to progressive intra-cranial disease and associated progressive neurological dysfunction. When a patient succumbed to progressive primary cancer, cause of death was categorized as primary cancer-related.
Statistical analyses were performed using SSPS version 12.0 (SPSS Institute, Inc., Chicago, IL, U.S.A.). Overall survivals were estimated using Kaplan-Meier methods. The Cox regression analysis was used to adjust the factors affecting survival. Variables found to be significantly associated with survival by univariate analyses were subjected to multivariate analyses. The chi-square tests were used to compare the two groups in terms of baseline characteristics. Results were considered significant when P values were less than 0.05.
Table 1 details patient characteristics and Table 2 shows summary. There were 19 male (76%) and 6 female (24%) patients of mean age 59.1 yr (range 39-71). The types of primary cancer follows; 15 (60%) NSCLC, 6 (24%) colon cancers, 1 (4%) breast cancer, 1 (4%) renal cell carcinoma, 1 (4%) ovarian cancer, and 1 (4%) esophageal cancer. In term of primary tumors without considering BM, 3 patients (12%) had a tumor stage of IIB, 3 (12%) a tumor stage of IIIB and 19 (76%) had a tumor stage of IV. Nineteen patients (76%) had extra-cranial metastasis. Eleven of the 25 primary cancers had a well-controlled status (44%), and 14 did not (56%). Twelve patients (48%) had a KPS score of <70 and 13 patients (52%) had a KPS score ≥70. Only one patient (4%) was of RPA class 1, 11 (44%) were of RPA class 2, and 13 (52%) were of RPA class 3.
As was expected, these 25 patients who survived for less than 3 months after craniotomy had higher rates of extra-cranial metastasis, progressive primary disease, a KPS score of <70, and RPA class 3 than all the patients who underwent brain metastatectomy during same period at the same institutes (P<0.05). These factors were associated with survival in the univariate analysis using Cox regression model. But, in the multivariate analysis, there was no statistical significance in the extracranial metastasis (P=0.067). Table 6 shows the relationship between survival and clinical factors.
Table 3 shows the detailed clinical features of brain lesions and Table 4 provides a summary. Brain lesions were <3 cm in 13 patients (52%) and ≥3 cm in 12 (48%). Twenty BMs (80%) were located in the supratentorial region and 5 (20%) in the infratentorial region. Two tumors (8%) were of functional grade 1, 10 (40%) tumors in functional grade 2, and the other 13 (52%) in functional grade 3. Six patients (24%) experienced synchronous development and 19 (76%) metachronous development.
Furthermore, patients who survived for less than 3 months were more likely to have a BM of <30 mm in size and to be of functional grade 3 than all the patients who underwent brain metastatectomy during the same period at the same institutes. Functional grade of BM location had statistical significance on survival in the univariate analysis (P=0.033) but did not in the multivariate analysis after multi-factor adjustment (P=0.078). Other features of BM did not have an influence of the survival in the univariate and multivariate (Table 7).
Table 5 details the patients who received adjuvant and neoadjuvant therapy. Nineteen patients (76%) did not receive any treatment for primary systemic cancer or BM before metastatectomy. One patient (4%) received systemic chemotherapy alone and another received brain irradiation alone before brain metastatectomy. Four patients (16%) received both. After metastatectomy, 12 patients (48%) did not receive adjuvant therapy, 7 patients (28%) received chemotherapy alone, 4 (16%) received brain irradiation alone, and the remaining 2 (8%) received both modalities after brain surgery.
Of the six patients who experienced synchronous BM development, two patients received neoadjuvant therapy before brain metastatectomy. One received neoadjuvant brain radiotherapy after BM diagnosis but before craniotomy, and the other received neoadjuvant chemotherapy and radiotherapy instead of immediate brain metastatectomy.
Patients who survived for less than 3 months were less likely to receive neoadjuvant and adjuvant therapy than all the patients who underwent brain metastatectomy during same period. But in the Cox regression analysis (Table 6), neoadjuvant (P=0.034) and adjuvant treatment (P=0.005) had statistically significant influences on the survival in the univariate analysis. But after multi-factor adjustment in the multivariate analysis, adjuvant treatment alone had statistical association with survival; the Hazard ratio of survival for the patients who did not received adjuvant therapy versus the patients who received adjuvant treatment was 2.89 (95% confidence interval, 1.16-5.26; P=0.023).
Fig. 1 details survivals. Mean survival after date of primary cancer diagnosis was 9.90 months (range, 2.30-30.70); 4.25 months (range, 2.30-8.47) in those that experienced synchronous BM development, and 13.4 months (range, 5.57-30.70) in those that experienced metachronous BM development.
Mean survival after craniotomy was 2.06 months (range, 0.67-2.93); 2.20 months (range, 1.50-2.60) in those that experienced synchronous BM development, and 2.02 months (range, 0.67-2.93) in those that experienced metachronous BM development.
Mean time-interval between the date of primary cancer diagnosis and the date of BM diagnosis was 7.54 months (range, 0.00-27.67); and 0.40 months (range, 0.00-1.60) in those that experienced synchronous BM development, and 9.79 months (range, 3.60-27.67) in those that experienced metachronous BM development.
Mean time-interval between the date of BM diagnosis and craniotomy was 1.62 months (range, 0.00-9.57); and 1.26 months (range, 0.00-5.53) in those that experienced synchronous BM development, and 1.73 months (range, 0.00-9.57) in those that experienced metachronous BM development.
Sixteen of the 25 patients died from primary cancer progression; multiple organ failures due to multiple metastases, which included malignant pleural effusion, neoplastic pneumonitis, renal failure, adrenal failures, and hepatic failures. Three patients succumbed to direct CNS-related progression; multiple distant metastasis or leptomeningeal metastases. Six patients suffered from systemic complications, e.g., pneumonia or sepsis, due indirectly to immobilization due to neurological dysfunction after brain surgery or brain irradiation or from reduced immunity due to chemotherapy. Table 8 details causes of death by adjuvant therapy modality.
The prognosis of BM patients is poor, and if untreated, its natural course involves progressive neurological deterioration with a median survival time of 1-2 months (10). However, surgical removal of a solitary BM prolongs survival and improves quality of life (11), although median overall survival for patients that undergo BM resection is only 4-7.7 months (10). Nevertheless, patients with metachronous BM have a substantially better prognosis after successful brain metastatectomy with estimated 5-yr survivals of 21-45% (12, 13). But, in synchronous BM patients, reports have shown considerably dismal results after resection of the BM (14, 15). Five-year survival rates of only 5-10% with a median survival of less than 10 months have been reported for synchronous BM patients treated by brain metastatectomy alone (16).
Occasionally, BM has life-threatening mass effects and causes focal neurological deficits that may considerably decrease functional grade. Thus, debulking can provide immediate symptom relief and improve patient quality of life. However, despite the benefits of surgical resection, surgical candidates cannot be defined clearly. The traditional criteria used to select patients who will benefit from surgery include a good physical function (as assessed using KPS scores), a single and surgically accessible metastasis, and stable or absent extra-cranial metastases. Allen K. Sills (17) recommended that the following factors be evaluated when considering surgery in BM patients; a single tumor, surgical accessibility, good tumor localization, a young age, a KPS score of 70 or more, RPA class 1, control of extra-cranial disease, confirmation of tissue histology, need for immediate tumor debulking, large tumor size, an undiagnosed primary tumor, a long disease-free interval, and the absence of leptomeningeal involvement.
In the present study, as was expected, these patients who survived for less than 3 months after craniotomy tended to have more progressive primary cancer, higher rate of a KPS score of <70, RPA class 3, and extra-cranial metastases, such as, to the adrenal gland, bone, liver, or spine. In terms of size, although BM may be small, an eloquent location often leads to neurological dysfunction, and therefore, we decided to resect BM to improve neurological deficit, such as motor weakness. However, in these cases, most neurological deficits were not recovered after surgery. Therefore, these twelve patients (48%) could not received adjuvant therapy due to a deterioration of performance status, and then, primary cancers or brain disease progressed in these patients. Additionally, many studies have shown that radiosurgery such as Gamma Knife Radiosurgery or Cyberknife is effective at controlling BM and prolonged survival (18-21), especially in case of small sized lesions. In this study, although brain lesion was small in size, most of patients who suffered from severe cerebral edema with risk of impending brain herniation and acute neurological deteriorations without response to medical treatment were treated with surgical decompression in order to prevent CNS-related death.
In the patients who succumbed to systemic complications, causes of death were aspiration pneumonia or adult respiratory distress syndrome after post-surgical or post-irradiative hemiparesis or sepsis during chemotherapy. In fact, patients who could not be administered adjuvant therapy had a poorer condition before and after brain metastatectomy, such as postoperative progression of neurological dysfunction and a poor general condition. Therefore, they could not receive any further treatment, and for these reasons primary cancers progressed.
In terms of adjuvant therapy, Macchiarini et al. (22) showed that systemic chemotherapy is the most significant independent predictor of disease-free long-term survival after brain metastasectomy. Our result that adjuvant treatment had an influence on survival concur with that of Macchiarini et al. (22) Patchell et al. (23) demonstrated recurrence and neurological death is less likely in patients treated with brain metastatectomy with following whole brain radiotherapy. Determining critical candidates for brain metastatectomy can improve survival in some patients and provide an opportunity for further treatment. In fact, the status of systemic disease is extremely important since most patients who undergo surgery for brain metastases eventually succumb to systemic disease, often without BM recurrence (24). These days many authors appear to view systemic chemotherapy as an important therapeutic modality that offers therapeutic benefit to BM patients (25-28).
It is difficult to advise on the role of neoadjuvant therapy in cases of brain metastasis based on the findings of the present study. Some differences in the neoadjuvant therapy were found between patients who survived for less than 3 months after brain metastatectomy and all the patients who underwent brain metastatectomy during the same period at the same institutes. But, treatment before BM diagnosis was considered in all the patients who underwent brain metastatectomy, therefore, more patients were found to receive neoadjuvant therapy. It is a fact that many patients should experience primary cancer progression in brain during systemic therapy for primary cancer, but the exact number was not calculated during the present study. Anyway, it is less meaningful that more patients who survived for less than 3 months might not perform neoadjuvant therapy in this study.
However, in the Kaplan-Meier survival analysis, median survival times of patients in analyzed many factors of Cox regression model did not reach median value, which suggested either the need for longer follow-up periods or the need for larger number of patients.
We failed to identify a time of BM onset during the expected clinical course of systemic cancer. If the time of BM occurrence during an expected survival period could be predicted, we would be able to precisely calculate remaining survival times. For example, if a BM occurs in a patient who diagnosed lung cancer 3 yr ago, and his survival was expected to 5 yr at that time, his remnant survival is 2 yr now. In this patient, brain metastatectomy can help to guarantee remnant survival. But, if BM occurs at 5 yr after diagnosis of lung cancer in this patient, brain surgery cannot help secure survival.
However, in the present study, too few patients were enrolled to perform this assessment. Thus, more clinical data is required to enable us to predict the timing of BM during the clinical course after a primary cancer diagnosis.
In conclusion, although this study was limited in scope, we assessed the features of patients that survived for 3 months or less after surgical resection for single brain metastasis, and compared with all the patients who underwent brain metastatectomy during same period at the same institutes. Status of primary cancer, RPA class, and functional status of patients are critical factors when selecting surgical candidates. After brain metastatectomy, good functional status which enabled the patients to undergo appropriate adjuvant therapy might be important to prolong survival in BM patients. Neurosurgeons must perform brain metastatectomy so as not to cause neurological complications that may cause sufficient deterioration to prevent subsequent adjuvant therapy on the base of individual conditions.
Figures and Tables
Fig. 1
Survival and time-intervals during clinical course of patients who survived less than 3 months after brain metastatectomy.

Table 3
Characteristics of brain metastases of the patients who survived for less than 3 months after brain metastatectomy (BM)
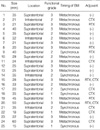
Table 6
Clinical factors of patients affecting survival in univariate and multivariate analysis using Cox regression model

References
2. Posner JB. Management of brain metastases. Rev Neurol (Paris). 1992. 148:477–487.
3. Wronski M, Arbit E, Burt M, Galicich JH. Survival after surgical treatment of brain metastases from lung cancer: a follow-up study of 231 patients treated between 1976 and 1991. J Neurosurg. 1995. 83:605–616.
4. Posner JB, Chernik NL. Intracranial metastases from systemic cancer. Adv Neurol. 1978. 19:579–592.
5. Lang Frederick F, Chang Eric L, Abis-Said Dima, Wildrick David M, Sawaya R. Youmans JR, editor. Metastatic brain tumors. Neurological Surgery. 2003. Vol 1:ed 5. Philadelphia: WB Saunders;1077–1097.
6. Central Nervous System Cancer. Version 1. NCCN Clinical Practice Guideline in Oncology. accessed Feb 27, 2008. Available at http://www.nccn.org/professionals/physician_gls/PDF/cns.pdf.
7. Karnofsky D, Abelmann W, Craver L. The use of the nitrogen mustards in the palliative treatment of carcinoma. Cancer. 1948. 1:634–656.
8. Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, McKenna WG, Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997. 37:745–751.

9. Sawaya R, Hammoud M, Schoppa D, Hess KR, Wu SZ, Shi WM, Wildrick DM. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998. 42:1044–1056.

10. Penel N, Brichet A, Prevost B, Duhamel A, Assaker R, Dubois F, Lafitte JJ. Prognostic factors of synchronous brain metastases from lung cancer. Lung Cancer. 2001. 33:143–154.
11. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS, Young B. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990. 322:494–500.

12. Hankins JR, Miller JE, Salcman M, Ferraro F, Green DC, Attar S, McLaughlin JS. Surgical management of lung cancer with solitary cerebral metastasis. Ann Thorac Surg. 1988. 46:24–28.

13. Magilligan DJ Jr, Duvernoy C, Malik G, Lewis JW Jr, Knighton R, Ausman JI. Surgical approach to lung cancer with solitary cerebral metastasis: twenty-five years' experience. Ann Thorac Surg. 1986. 42:360–364.

14. Mussi A, Pistolesi M, Lucchi M, Janni A, Chella A, Parenti G, Rossi G, Angeletti CA. Resection of single brain metastasis in non-small cell lung cancer: prognostic factors. J Thorac Cardiovasc Surg. 1996. 112:146–153.
15. Rossi NP, Zavala DC, VanGilder JC. A combined surgical approach to non-oat-cell pulmonary carcinoma with single cerebral metastasis. Respiration. 1987. 51:170–178.

16. Billing PS, Miller DL, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Surgical treatment of primary lung cancer with synchronous brain metastases. J Thorac Cardiovasc Surg. 2001. 122:548–553.

17. Sills AK. Current treatment approaches to surgery for brain metastases. Neurosurgery. 2005. 57(5):Suppl. S24–S32.

18. Gerosa M, Nicolato A, Severi F, Ferraresi P, Masotto B, Barone G, Foroni R, Piovan E, Pasoli A, Bricolo A. Gamma knife radiosurgery for intracranial metastases: from local tumor control to increased survival. Stereotact Funct Neurosurg. 1996. 66:Suppl 1. 184–192.

19. Hasegawa T, Kondziolka D, Flickinger JC, Germanwala A, Lunsford LD. Brain metastases treated with radiosurgery alone: An alternative to whole brain radiotherapy? Neurosurgery. 2003. 52:1318–1326.

20. Lutterbach J, Cyron D, Henne K, Ostertag CB. Radiosurgery followed by planned observation in patients with one to three brain metastases. Neurosurgery. 2003. 52:1066–1074.

21. Muacevic A, Kreth FW, Tonn JC, Wowra B. Stereotactic radiosurgery for multiple brain metastases from breast carcinoma. Cancer. 2004. 100:1705–1711.

22. Macchiarini P, Buonaguidi R, Hardin M, Mussi A, Angeletti CA. Results and prognostic factors of surgery in the management of non-small cell lung cancer with solitary brain metastasis. Cancer. 1991. 68:300–304.

23. Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, Markesbery WR, Foon KA, Young B. Postoperative radiotherapy in the treatment of single metastases to the brain; a randomized trial. JAMA. 1998. 280:1485–1489.

24. Lang FF, Sawaya R. Surgical treatment of metastatic brain tumors. Semin Surg Oncol. 1998. 14:53–63.

25. Postmus PE, Smit EF. Chemotherapy for brain metastases of lung cancer; a review. Ann Oncol. 1999. 10:753–759.

26. Soffietti R, Costanza A, Laguzzi E, Nobile M, Ruda R. Radiotherapy and chemotherapy of brain metastases. J Neurooncol. 2005. 75:31–42.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download