Abstract
The overexpression of X-linked inhibitor of apoptosis protein (XIAP), a member of IAP family protein, is intuitively expected to be associated with unfavorable clinical features in malignancies; however, there have been only a very limited number of studies reporting the clinical relevance of XIAP expression. This study was performed to investigate the prognostic relevance of XIAP expression in childhood acute myeloid leukemia (AML). In 53 children with de novo AML, the level of XIAP expression was determined by using quantitative reverse transcriptase-polymerase chain reaction and was analyzed with respect to the clinical characteristics at diagnosis and treatment outcomes. As a result, the XIAP expression was found to be higher in patients with extramedullary disease than in those without (P=0.014). In addition, XIAP overexpression (≥median expression) was associated with an unfavorable day 7 response to induction chemotherapy and also associated with a worse 3-yr relapsefree survival rate (52.7±20.9% vs. 85.9±14.8%, P=0.014). Multivariate analyses revealed that XIAP overexpression was an independent unfavorable prognostic factor for relapse-free survival (hazard ratio, 6.16; 95% confidence interval, 1.48-25.74; P=0.013). Collectively, XIAP overexpression may be used as an unfavorable prognostic marker in childhood AML.
Acute myeloid leukemia (AML) comprises a heterogeneous group of hematological malignancies that arise within the bone marrow precursors of the myeloid, monocyte, erythroid, and megakaryocytic cell lineages. AML makes up 15% to 25% of childhood leukemia and accounts for more than 30% of the deaths from leukemia. The predictors of favorable prognosis in AML include favorable cytogenetics such as t(15;17), inv(16) and t(8;21) (1-3), and a good response to induction chemotherapy (3-6).
Recently, the accumulation of knowledge on the molecular biology of malignancies has led to new diagnostic modalities to be incorporated into various diagnostic and therapeutic strategies (7, 8). One of these modalities is the quantitative reverse transcriptase-polymerase chain reaction (RT-PCR), which allows researchers to examine the expression patterns of a large number of genes at the RNA level. It would be possible to refine the current prognosis-based stratification systems if specific patterns of gene expression can be correlated with the prognosis in childhood AML.
Apoptosis is an active biological mechanism leading to programmed cell death. The up-regulation of antiapoptotic regulators would certainly be advantageous for tumor survival (9). Over the last decade, a complex network of pro- and antiapoptotic proteins, which strictly regulate the apoptosis pathways, has been revealed. In particular, a group of proteins known as the inhibitor of apoptosis proteins (IAPs) were identified (10, 11). The IAPs are a family of proteins that have one to three baculovirus IAP repeat (BIR) domains, and inhibit apoptosis by direct binding and inhibiting caspases. Studies investigating the expression of these molecules in acute leukemia have demonstrated that the expression of pro- or antiapoptotic regulators varies depending on the types of leukemias and individual patients' characteristics (12-23). These differences can be potentially important for the prediction of the response to treatment.
X-linked inhibitor of apoptosis protein (XIAP, MIM# 300079; baculoviral IAP repeat-containing 4, BIRC4; also known as hILP and MIHA) gene is located on the chromosome band Xq25 and encodes a cytoplasmic protein of 497 amino acids, 57 kDa (24). XIAP contains 3 tandem BIR domains and a really interesting new gene (RING) domain. It binds and inhibits caspases 3, 7, and 9, but it does not bind or inhibit caspase 8. Through its ability to inhibit apoptosis, XIAP is intuitively expected to be associated with unfavorable clinical features in malignant diseases; however, only a very limited number of clinical studies on the prognostic relevance of XIAP expression in AML, in particular, childhood AML are available to date (14, 15).
In this study, therefore, the authors analyzed the expression of XIAP in 53 children with de novo AML using quantitative RT-PCR to determine the possible relationship between the XIAP expression and the clinical features at diagnosis and treatment outcomes. As a result, XIAP overexpression was found to be strongly associated with both unfavorable early responses to induction chemotherapy and worse long-term treatment outcomes. This is the first study demonstrating that XIAP overexpression is an independent unfavorable prognostic factor for relapse-free survival (RFS) in childhood AML.
Children younger than 15 yr of age who were newly diagnosed with de novo AML from July 2000 to April 2006 were enrolled in this study. The diagnosis of AML was made based on a morphologic assessment of the Wright-Giemsa-stained smears of the bone marrow aspirates along with special stains and immunophenotyping by flow cytometry. Laboratory investigation included conventional and molecular cytogenetic analyses.
All patients were treated according to the modified Korean Biologic Response Modifier Society (KBRMS) protocol (Table 1). The patients with AML other than acute promyelocytic leukemia (APL) first received 10 days of induction chemotherapy, in which the dose of behenoyl cytosine arabinoside (BH-AC) for the last 3 days was modified according to the bone marrow response on day 7. Discontinuation of the chemotherapy was allowed before the completion of the induction regimen in patients who experienced sepsis syndrome if at least 7 days of induction chemotherapy had been provided. If complete remission (CR) was not achieved after the primary induction chemotherapy regimen, an additional course of induction chemotherapy using high-dose cytosine arabinoside was administered. Once CR had been achieved, patients with an appropriate stem cell donor received consolidation chemotherapy until the hematopoietic stem cell transplantation. An entire course of consolidation chemotherapy was administered in patients without an appropriate stem cell donor and in patients with Down syndrome.
For patients with APL, the induction regimen was composed of all-trans-retinoic acid (ATRA, 45 mg/m2/day from day 0 until CR was achieved) and idarubicin (12 mg/m2/day on days 1, 3, 5, and 7). Once CR had been achieved, the patients received 3 courses of consolidation chemotherapy (1st, 2nd, and 4th consolidation chemotherapy according to the modified KBRMS protocol) along with daily ATRA (45 mg/m2/day). The patients then received the maintenance chemotherapy (ATRA 45 mg/m2/day for 15 days every 12 weeks, 6-mercaptopurine 50 mg/m2/day daily and methotrexate 10 mg/m2/day weekly) over a 2-yr period.
The Institutional Review Board of Samsung Medical Center approved this study, and informed consent was obtained from parents or guardians for both the laboratory studies and treatment.
Mononuclear cells (MNCs) were isolated from 2 mL of bone marrow aspirate at diagnosis by ficoll density gradient centrifugation. The total RNA was extracted from the MNCs using a QIAamp RNA Blood kit (Qiagen, Hilden, Germany) according to the manufacturer's protocol. After treatment with DNA-free (Ambion, Austin, TX, U.S.A.) to remove the chromosomal DNA, the complementary DNA (cDNA) was synthesized using oligo (dT) 15-mer primer by Super-Script III Reverse Transcriptase (Invitrogen, Carlsbad, CA, U.S.A.) and stored at -20℃ until use. The mRNA expression levels of XIAP and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were measured by quantitative RT-PCR using ABI PRISM 7000 Sequence Detector System (Applied Biosystems, Foster City, CA, U.S.A.). The quantitative RT-PCR amplification was performed using the predeveloped Assay-on-Demand Gene Expression Set for the XIAP gene (Hs01597783_m1; GenBank accession no. NM_001167.2; Applied Biosystems) and TaqMan GAPDH Control Reagents (Applied Biosystems) for the GAPDH gene in combination with the TaqMan Universal PCR Master Mix (Applied Biosystems). All reactions were performed in triplicate using 20 µL samples containing 50 ng cDNA. The reaction protocol used involved heating for 2 min at 50℃ and 10 min at 95℃, followed by 40 cycles of amplification (15 sec at 95℃ and 1 min at 60℃). Analysis was performed using ABI PRISM 7000 Sequence Detection software (Applied Biosystems). The levels of XIAP expression in unknown samples were calculated as a ratio of XIAP to GAPDH. The levels of XIAP and GAPDH mRNA expression were quantified using the standard curves generated from known serial dilutions of the standard RNA obtained from A549 cells by assuming a linear relationship between the first cycle number, at which the fluorescence signal significantly increased (Ct value), and the logarithm of the starting quantity. A negative control without a template was included in each experiment.
Cytotoxicity assay was done to evaluate the ex vivo susceptibility of leukemic blasts to apoptotic stimuli provided by chemotherapeutic agents. Survival of leukemic blasts after the exposure to etoposide was assessed by measuring the conversion of the trazolium salt WST-8 to formazan according to the manufacturer's instructions (Cell Counting Kit-8; Dojindo, Kumamoto, Japan) (12, 25). Briefly, leukemic cells (1.5×105 cells/well) in RPMI-1640 media supplemented with 10% fetal bovine serum and penicillin were plated onto 96-well plates. The cells were cultured in triplicate wells with 1 mM etoposide for 24, 48 and 72 hr in a humidified atmosphere containing 5% CO2 at 37℃. At the end of each time point, 10 µL WST-8 was added to each well, and the plates were incubated for additional 4 hr at 37℃ to convert WST-8 into formazan. The absorbance of each plate was measured at 450 nm and 600 nm. The absorbance at 450 nm represents a direct correlation with the cell number in this analysis. The results were expressed as the percentage of the absorbance of control (untreated and serial diluted) cells. The control cell number was assessed by trypan blue exclusion (final concentration, 0.2%) for 5 min using a hemocytometer.
Differences in the XIAP expression level with respect to the clinical factors at diagnosis (i.e., sex, age, leukocyte count, the presence or absence of extramedullary disease, French-American-British [FAB] classification [26], structural cytogenetic abnormalities and FLT3 gene mutations) and treatment outcomes (day 7 response to induction chemotherapy, induction of remission with the primary induction chemotherapy regimen and occurrence of relapse) were analyzed using the Mann-Whitney U test. The expression levels are presented as the median values along with the ranges. The patients were categorized into 2 groups according to the level of XIAP expression (≥median vs. <median). The proportion of patients with an unfavorable day 7 response and patients in whom leukemia relapsed in the 2 groups of patients was compared using the Pearson chi-square test. Event-free survival (EFS) and relapse-free survival (RFS) rates along with 95% confidence interval (CI) were estimated using the Kaplan-Meier method. An event was defined as the occurrence of leukemia relapse or treatment-related death. The differences in the survival rates between 2 groups according to the XIAP expression levels (≥median vs. <median) were compared using the log-rank test. Multivariate analyses comprising clinical factors for RFS was performed using the Cox regression analysis. Statistical significance was accepted when the P values were less than 0.05.
Fifty-three children were enrolled in this study. Clinical characteristics of the patients are presented in Table 2. The median age at diagnosis among the 53 patients (32 boys and 21 girls) was 81 (range 2-179) months, and their median leukocyte count at diagnosis was 20,460/µL (range 440-345,950/µL). When the disease was classified according to the FAB classification, 1 patient had AML M0, 28 had M1/M2, 5 had M3, 13 had M4/M5 and 6 had M7. Three patients had Down syndrome, and one of them had FAB M7 subtype. The common structural abnormalities identified by conventional chromosomal analysis, fluorescence in situ hybridization examination, or both were t(8;21) in 11 patients, t(15;17) in 5 patients, 11q23 rearrangement in 11 patients and inv (16) in 2 patients. Among 12 patients with extramedullary disease, 11 patients had chloroma (scalp in 5 patients, orbit in 3 patients and skin in 3 patients), and 1 patient had a central nervous system involvement of leukemic cells. The FLT3 gene mutation was found in 8 patients among 50 patients tested, which revealed 4 patients had internal tandem duplications (ITD) and 4 patients had point mutations in the tyrosine kinase domain (TKD). Forty-one (77.4%) patients achieved CR after primary induction chemotherapy. Twenty-nine patients received allogeneic stem cell transplant from 6 related and 23 unrelated donors (14 bone marrow, 11 cord blood and 4 peripheral blood stem cells) at first CR. The median proportion of bone marrow leukemic blasts at diagnosis was 73 (range 25-98)%. The proportion of patients with ≥50% of bone marrow leukemic blasts at diagnosis was 71.7%. There was no difference in the proportion of bone marrow leukemic blasts between the patients with XIAP overexpression (≥median) and those without (P=0.722).
XIAP expressions with respect to the clinical features at diagnosis are presented in Table 2. The median expression level of XIAP in 53 patients was 1.40 (range 0.00-2,867.19). In 4 patients, XIAP expression was not detected. The level of XIAP expression was higher in patients with extramedullary disease than in those without (P=0.014; Fig. 1A). The level of XIAP expression was higher in patients with a high leukocyte count (≥20,000/µL) and patients with FLT3-ITD/TKD mutation than in those without, but the difference was not significant (Fig. 1B, C). The level of XIAP expression in 3 patients with Down syndrome was not significantly different from that in the remaining patients. There was no difference in the level of XIAP expression between FAB M2/M3 and M4/M5 subtype or between favorable and intermediate/unfavorable cytogenetic abnormalities (3). The levels of XIAP expression in patients with t(15;17), t(8;21) or inv (16) were not different from those in patients with other cytogenetic abnormalities (Fig. 1D).
The levels of XIAP expression with respect to responses to chemotherapy are also presented in Table 2. XIAP overexpression (≥median) was associated with a worse early response to induction chemotherapy. The level of XIAP expression was higher in patients with an unfavorable day 7 response (defined as leukemic blasts >5% on the bone marrow aspirate on day 7, the presence of a leukemic cell cluster(s) on the bone marrow biopsy section on day 7 or persistence of circulating leukemic blasts in the peripheral blood on day 7) than in those with a favorable day 7 response (absence of any of the three aforementioned findings indicating an unfavorable response) (P=0.006; Fig. 2A). Similarly, the proportion of patients with an unfavorable day 7 response was higher in patients with XIAP overexpression (≥median) than in those without (40.9% vs. 15.0%, P=0.063; Fig. 2B). In the cytotoxicity assay, the percentage of surviving leukemic blasts after 24 hr culture with etoposide was higher in patients with high XIAP expression (upper one-third) than in patients with low XIAP expression (lower one-third) (P=0.054; Fig. 2C). The level of XIAP expression was also higher in the patients who did not achieve CR after primary induction chemotherapy than in those who did; however, the difference was not significant. Similarly, the induction failure rate with primary induction chemotherapy regimen due to poor response was higher in patients with XIAP overexpression (6 out of 27) than in those without (2 out of 26), albeit without a statistical significance (P=0.138).
The leukemia relapsed in 14 patients, and treatment-related mortality occurred in 7 patients. The median follow-up duration among 32 live patients was 42 (range 20-88) months. The 3-yr RFS and EFS rates (±95% CI) in all 53 patients were 69.0±13.6% and 58.9±13.6%, respectively. The 3-yr RFS rate in 33 patients (other than AML, M3 or Down syndrome) who were assigned to receive allogeneic transplantation at first CR was slightly higher than in 6 patients who were assigned to receive continuous chemotherapy alone or autologous transplantation at first CR, but it was not significant (69.7±15.7% vs. 60.0±42.9%, P=0.981). The level of XIAP expression was higher in the patients who experienced relapse than in those who had been in continuous CR (P=0.015; Fig. 3A). Leukemia relapse was more frequent in patients with XIAP overexpression (11 out of 27) than in those without (3 out of 26) (P=0.016; Fig. 3B). The 3-yr RFS rate was lower in patients with XIAP overexpression than in those without (52.7±20.9% vs. 85.9±14.8%, P=0.014; Fig. 3C). Similarly, the 3-yr RFS rate after allogeneic transplantation was lower in patients with XIAP overexpression than in those without, although it was not significant (74.6±16.2% vs. 87.5±25.1%, P=0.339; Fig. 3D). Multivariate analyses for RFS revealed that XIAP overexpression was an independent unfavorable prognostic factor with a statistical significance in childhood AML (hazard ratio, 6.16; 95% CI, 1.48-25.74; P=0.013; Table 3).
A variety of anti-apoptotic proteins are expressed in different tumors, and their expression levels may be related to the unfavorable features at diagnosis, a poor response to treatment or both. However, the clinical relevance of these biological regulators remains largely elusive, and particularly, there have been no studies on the prognostic relevance of XIAP expression in childhood AML to date, except for the only study by Tamm et al. (15) who demonstrated that a high level of XIAP expression correlated with a poorer overall survival in childhood AML. From this context, the present study was performed to investigate the possible relationship between XIAP expression and the clinical features in childhood de novo AML.
In the present study, the level of XIAP expression was found to be associated with the clinical characteristics at diagnosis. The level of XIAP expression was higher in patients with extramedullary disease than in those without. In addition, the level of XIAP expression was higher in patients with a high leukocyte count and patients with FLT3-ITD/TKD mutation than in those without, although the differences were not statistically significant. However, there was no significant difference in the level of XIAP expression according to cytogenetic abnormalities (favorable vs. intermediate/unfavorable), which are known to be the most significant prognostic factor in AML (3). These findings suggest that XIAP expression is independent of cytogenetic abnormalities in childhood AML.
The early response to induction chemotherapy has proven to play an important role in children with acute lymphoblastic leukemia (ALL) (27-29). In childhood ALL, the failure of achieving blast clearance from the bone marrow aspirates after 1 or 2 weeks of induction chemotherapy (27, 28) or the persistence of circulating blasts after 1 week of multi-agent chemotherapy (29) indicates a poor prognosis. It is likely that the initial response to induction chemotherapy may also be predictive of the outcome in childhood AML, although there are currently limited data available to draw a definite conclusion. Some studies evaluated the response to induction chemotherapy by assessing the degree of residual leukemic infiltration in the bone marrow after 6 or 14 days of induction chemotherapy (4-6). In the present study, the early response to induction chemotherapy was evaluated on day 7, and the unfavorable day 7 response was strongly associated with a worse 3-yr RFS (80.9±15.2% vs. 38.1±28.8%, P=0.008). Here again, it is of note that the level of XIAP expression was significantly higher in patients with an unfavorable day 7 response than in those without. Similarly, the proportion of patients with an unfavorable day 7 response was higher in patients with XIAP overexpression (≥median) than in those without. These findings were in line with the result of in vitro cytotoxicity assay. In addition, the frequency of induction failure after primary induction chemotherapy due to poor response was higher in patients with XIAP overexpression than in those without, albeit without a statistical significance. These findings suggest that XIAP overexpression is associated with a worse early response to induction chemotherapy. The level of XIAP expression was higher in patients who experienced relapse than in patients who had been in continuous CR. Similarly, the frequency of relapse was higher in patients with XIAP overexpression than in those without. As a result, the 3-yr RFS rate was lower in patients with XIAP overexpression than in those without. In the multivariate analyses, XIAP overexpression was an independent unfavorable prognostic factor for RFS. Collectively, it was suggested that XIAP overexpression is associated with delayed blast clearance from the bone marrow or peripheral blood and the resistance of blasts to apoptotic stimuli provided by the chemotherapeutic agents, eventually leading to a worse RFS.
The findings in the present study are basically in line with those by Tamm et al. (15) in that XIAP overexpression is associated with a worse outcome. However, there are a few important differences between the two studies. First, there was no difference in the level of XIAP expression between FAB M2/M3 and M4/M5 subtype in the present study, while the level of XIAP expression was higher in M4/M5 subtype than in M2/M3 subtype according to the report by Tamm et al. (15). Second, while Tamm et al. (15) could detect XIAP expression only in patients with intermediate/unfavorable cytogenetics, we detected XIAP expression even in patients with favorable cytogenetics and there was no difference in the level of XIAP expression between favorable and intermediate/unfavorable cytogenetics. Third, most importantly, the level of XIAP expression was higher in patients who experienced relapse than in those in continuous CR in this study, while Tamm et al. (15) could not find a significant difference in XIAP expression between the two groups of patients. As a result, they could not find a difference in RFS according to the level of XIAP expression although the high level of XIAP expression was associated with a worse overall survival rate. These differences between the two studies may be partly explained by the difference in methods to measure XIAP expression (quantitative RT-PCR vs. Western blot). In addition, as Tamm et al. (15) pointed out in their report, selection bias toward samples from patients with high leukocyte counts during cryopreservation of samples and alteration in protein expression by freezing and DMSO might have resulted in different findings from our study.
The IAP family proteins inhibit the apoptosis induced by a variety of stimuli including chemotherapeutic agents, and therefore, their overexpression is expected to be associated with unfavorable clinical features in a variety of malignancies including AML. However, the clinical significance of IAP overexpression in acute leukemia is not completely consistent with what has been expected from previous in vitro studies. For example, IAP overexpression was not always associated with the unfavorable clinical features in acute leukemia (16). Furthermore, it was recently reported that the high expression of Livin, also a member of IAP family proteins, is an independent favorable prognostic factor in childhood ALL (12). This suggests that the role of IAP in leukemogenesis or in the maintenance of leukemic cells might be different from what has been previously recognized. However, the findings from this study demonstrated that the clinical significance of XIAP overexpression in childhood AML is consistent with what has been previously recognized from in vitro studies.
In conclusion, this is the first report demonstrating that XIAP overexpression is associated with a worse early response to induction chemotherapy and, eventually, is an independent unfavorable prognostic factor for RFS in childhood AML. Admitting that the number of cases and the follow-up period in this study were limited, we hope this study would prompt other scientists to investigate the implications of XIAP overexpression in childhood AML to confirm our observations in a larger series of patients. Through validation by further studies, XIAP overexpression may be used as an unfavorable prognostic marker in childhood AML.
Figures and Tables
Fig. 1
The level of XIAP expression with respect to the clinical characteristics at diagnosis. The level of XIAP expression was higher in patients with extramedullary disease than in those without (A). The level of XIAP expression was higher in patients with high leukocyte counts (≥20,000/µL) (B) and patients with FLT3-ITD/TKD mutation than in those without (C), but the differences were not statistically significant. The expression was not differed by cytogenetic abnormalities (D).

Fig. 2
XIAP overexpression: association with a worse early response to induction chemotherapy. The level of XIAP expression was higher in patients with an unfavorable day 7 response (defined as leukemic blasts > 5% on the bone marrow aspirate on day 7, the presence of a leukemic cell cluster[s] on the bone marrow biopsy section on day 7 or persistence of circulating leukemic blasts in the peripheral blood on day 7) than in those with a favorable day 7 response (absence of any of the above findings indicating an unfavorable response) (A). The proportion of patients with an unfavorable day 7 response was higher in patients with high XIAP expression (≥median) than in patients with low XIAP expression (<median) (B). In the cytotoxicity assay, the percentage of surviving leukemic blasts after 24 hr culture with etoposide was higher in patients with high XIAP expression (upper one-third, n=2) than in those with low XIAP expression (lower one-third, n=2) (C).
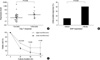
Fig. 3
XIAP overexpression: association with a worse long-term relapse-free survival (RFS) rate. The level of XIAP expression was higher in the patients who experienced relapse than in those who had been in continuous CR (complete remission) (A). The relapse of disease was more frequent in patients with XIAP overexpression (11 out of 27) than in those without (3 out of 26) (B). Similarly, the 3-yr RFS rate was lower in patients with XIAP overexpression than in those without (52.7±20.9% vs. 85.9±14.8%) (C).

Table 2
The levels of XIAP expression and the treatment outcomes with respect to the clinical factors of patients

Differences in the level of XIAP expression were analyzed using the Mann-Whitney U test. Statistical significance was accepted when the P values were less than 0.05.
*Day 7 bone marrow examination was not performed in 11 patients (5 patients with APL, 3 patients with severe infection and 3 patients who experienced early death); †Four patients died from toxicity during induction chemotherapy.
ACKNOWLEDGMENTS
We thank the residents and nurses who cared for the patients, and without whom this study would not have been possible.
References
1. Chang M, Raimondi SC, Ravindranath Y, Carroll AJ, Camitta B, Gresik MV, Steuber CP, Weinstein H. Prognostic factors in children and adolescents with acute myeloid leukemia (excluding children with Down syndrome and acute promyelocytic leukemia): univariate and recursive partitioning analysis of patients treated on Pediatric Oncology Group (POG) Study 8821. Leukemia. 2000. 14:1201–1207.

2. Grimwade D, Walker H, Harrison G, Oliver F, Chatters S, Harrison CJ, Wheatley K, Burnett AK, Goldstone AH. The predictive value of hierarchical cytogenetic classification in older adults with acute myeloid leukemia (AML): analysis of 1065 patients entered into the United Kingdom Medical Research Council AML11 trial. Blood. 2001. 98:1312–1320.

3. Wheatley K, Burnett AK, Goldstone AH, Gray RG, Hann IM, Harrison CJ, Rees JK, Stevens RF, Walker H. A simple, robust, validated and highly predictive index for the determination of risk-directed therapy in acute myeloid leukaemia derived from the MRC AML 10 trial. United Kingdom Medical Research Council's Adult and Childhood Leukaemia Working Parties. Br J Haematol. 1999. 107:69–79.
4. Browman G, Preisler H, Raza A, Syracuse K, Azarnia N, Benger A, Chervenick P, D'Arrigo P, Doeblin T, Goldberg J. Use of the day 6 bone marrow to alter remission induction therapy in patients with acute myeloid leukaemia: a leukemia intergroup study. Br J Haematol. 1989. 71:493–497.

5. Peters WG, Willemze R, Zwaan FE, Colly LP. Day-6 bone marrow aspirate for the prediction of response to remission induction therapy for acute myelogenous leukaemia. Blut. 1988. 57:91–95.

6. Kern W, Haferlach T, Schoch C, Loffler H, Gassmann W, Heinecke A, Sauerland MC, Berdel W, Buchner T, Hiddemann W. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete remission and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003. 101:64–70.

7. Golub TR. Genomic approaches to the pathogenesis of hematologic malignancy. Curr Opin Hematol. 2001. 8:252–261.

9. Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood. 2001. 98:2603–2614.

10. Yang YL, Li XM. The IAP family: endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000. 10:169–177.

11. Nachmias B, Ashhab Y, Ben-Yehuda D. The inhibitor of apoptosis protein family (IAPs): an emerging therapeutic target in cancer. Semin Cancer Biol. 2004. 14:231–243.

12. Choi J, Hwang YK, Sung KW, Lee SH, Yoo KH, Jung HL, Koo HH, Kim HJ, Kang HJ, Shin HY, Ahn HS. Expression of Livin, an antiapoptotic protein, is an independent favorable prognostic factor in childhood acute lymphoblastic leukemia. Blood. 2007. 109:471–477.

13. Choi J, Hwang YK, Sung KW, Kim DH, Yoo KH, Jung HL, Koo HH. Aven overexpression: association with poor prognosis in childhood acute lymphoblastic leuk. Leuk Res. 2006. 30:1019–1025.
14. Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, Andreeff M, Reed JC. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000. 6:1796–1803.
15. Tamm I, Richter S, Oltersdorf D, Creutzig U, Harbott J, Scholz F, Karawajew L, Ludwig WD, Wuchter C. High expression levels of x-linked inhibitor of apoptosis protein and survivin correlate with poor overall survival in childhood de novo acute myeloid leukemia. Clin Cancer Res. 2004. 10:3737–3744.

16. Wrzesien-Kus A, Smolewski P, Sobczak-Pluta A, Wierzbowska A, Robak T. The inhibitor of apoptosis protein family and its antagonists in acute leukemias. Apoptosis. 2004. 9:705–715.

17. Nakagawa Y, Yamaguchi S, Hasegawa M, Nemoto T, Inoue M, Suzuki K, Hirokawa K, Kitagawa M. Differential expression of survivin in bone marrow cells from patients with acute lymphocytic leukemia and chronic lymphocytic leukemia. Leuk Res. 2004. 28:487–494.

18. Yamamoto K, Abe S, Nakagawa Y, Suzuki K, Hasegawa M, Inoue M, Kurata M, Hirokawa K, Kitagawa M. Expression of IAP family proteins in myelodysplastic syndromes transforming to overt leukemia. Leuk Res. 2004. 28:1203–1211.

19. Wuchter C, Richter S, Oltersdorf D, Karawajew L, Ludwig WD, Tamm I. Differences in the expression pattern of apoptosis-related molecules between childhood and adult de novo acute myeloid leukemia. Haematologica. 2004. 89:363–364.
20. Campos L, Sabido O, Sebban C, Charrin C, Bertheas MF, Fiere D, Guyotat D. Expression of BCL-2 proto-oncogene in adult acute lymphoblastic leukemia. Leukemia. 1996. 10:434–438.
21. Coustan-Smith E, Kitanaka A, Pui CH, McNinch L, Evans WE, Raimondi SC, Behm FG, Arico M, Campana D. Clinical relevance of BCL-2 overexpression in childhood acute lymphoblastic leukemia. Blood. 1996. 87:1140–1146.

22. Hogarth LA, Hall AG. Increased BAX expression is associated with an increased risk of relapse in childhood acute lymphocytic leukemia. Blood. 1999. 93:2671–2678.

23. Prokop A, Wieder T, Sturm I, Essmann F, Seeger K, Wuchter C, Ludwig WD, Henze G, Dorken B, Daniel PT. Relapse in childhood acute lymphoblastic leukemia is associated with a decrease of the Bax/Bcl-2 ratio and loss of spontaneous caspase-3 processing in vivo. Leukemia. 2000. 14:1606–1613.

24. Rajcan-Separovic E, Liston P, Lefebvre C, Korneluk RG. Assignment of human inhibitor of apoptosis protein (IAP) genes xiap, hiap-1, hiap-2 to chromosomes Xq25 and 11q22-q23 by fluorescence in situ hybridization. Genomics. 1996. 37:404–406.
25. Kaspers GJ, Veerman AJ, Pieters R, Van Zantwijk CH, Smets LA, Van Wering ER, Van Der Does-Van Den Berg A. In vitro cellular drug resistance and prognosis in newly diagnosed childhood acute lymphoblastic leukemia. Blood. 1997. 90:2723–2729.

26. Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, Sultan C. Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French-American-British Cooperative Group. Ann Intern Med. 1985. 103:620–625.
27. Gaynon PS, Desai AA, Bostrom BC, Hutchinson RJ, Lange BJ, Nachman JB, Reaman GH, Sather HN, Steinherz PG, Trigg ME, Tubergen DG, Uckun FM. Early response to therapy and outcome in childhood acute lymphoblastic leukemia: a review. Cancer. 1997. 80:1717–1726.
28. Nachman J, Sather HN, Gaynon PS, Lukens JN, Wolff L, Trigg ME. Augmented Berlin-Frankfurt-Munster therapy abrogates the adverse prognostic significance of slow early response to induction chemotherapy for children and adolescents with acute lymphoblastic leukemia and unfavorable presenting features: a report from the Children's Cancer Group. J Clin Oncol. 1997. 15:2222–2230.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download