Abstract
A 65-yr-old woman presented 17 yr status post-hysterectomy with bilateral ovarian salpingo-oophorectomy, attributable to ovarian cancer. She was admitted to our hospital, with multiple cystic liver masses and multiple large seeded masses in her abdomen and pelvic cavity. Histological examination of the pelvic masses demonstrated granulosa cell tumors. After two courses of systemic combination chemotherapy, with paclitaxel and carboplatin, the masses in the abdomen and pelvic cavity increased, and debulking surgery also failed because of peritoneal dissemination with severe adhesion. Finally, she underwent palliative radiotherapy for only the pelvic masses obstructing the urinary and GI tracts, and monthly hormonal therapy with a gonadotrophin-releasing hormone agonist; leuprorelin 3.75 mg IM. Subsequently, multiple masses beyond the range of the radiation as well as those within the radiotherapy field partially decreased. This partial response had been maintained for more than 8 months as of the last follow-up visit. Owing to its long and indolent course and the low metabolic rate of the tumors, advanced or recurrent granulosa cell tumor (GCT) requires treatment options beyond chemotherapy, surgery, and radiotherapy. Hormonal agents may provide another treatment option for advanced or recurrent GCT in those who are not candidates for surgery, chemotherapy, or radiotherapy.
Sex cord-stromal tumor of the ovary is an uncommon neoplasm that accounts for approximately 7% of all malignant ovarian neoplasms (1, 2). Granulosa cell tumors (GCTs) are derived from granulosa cells, a hormonally active component of the ovarian stroma responsible for estradiol production. Their rarity has limited our understanding of the natural history and management of this cancer. The usual natural history of GCTs is indolent, with a very favorable long-term prognosis; however, relapses tend to occur, typically many years after the original diagnosis.
There is no standard approach to the management of relapsed GCT, and a combination of several modalities, such as surgery followed by radiation or chemotherapy, have been associated with prolonged disease-free survival (3, 4). However, owing to the indolent and long history of GCTs, additional therapeutic approaches, such as hormonal therapy, are required. Although a considerable rationale exists for the use of hormonal therapy in GCTs, clinical experience with this approach is extremely limited.
Here, we present a case in which a gonadotropin-releasing hormone (GnRH) agonist was successfully used to treat recurrent disease in a woman with a granulosa cell tumor, which had failed to respond to systemic chemotherapy and surgery.
A 65-yr-old multiparous woman presented 17 yr status post-hysterectomy with bilateral ovarian salpingo-oophorectomy, attributable to ovarian cancer. She had not received adjuvant therapy because of intolerance and she had not undergone regular follow-up monitoring. After 7 yr, she visited another hospital for abdominal pain, and she found that she had relapsed, developing multiple liver masses with granulosa cell tumors, and underwent three cycles of transarterial chemotherapy with cisplatin (100 mg/m2) without systemic chemotherapy. The response to transarterial chemotherapy was not fully determined. We could not get additional information regarding her medical information, because of limitations at the other institute and the long time gap. Ten years after the transarterial chemotherapy, without any regular follow-up monitoring, she was admitted via our emergency room because of abdominal pain and hematuria. She presented with multiple cystic liver masses, multiple large seeded masses in the abdomen and pelvic cavity, and hydronephrosis of her left kidney (Fig. 1A). The masses were recognized as hypometabolic by positron emission tomography-computed tomography (PET-CT) (Fig. 1B). Histological examination of the pelvic masses demonstrated granulosa cell tumors that were negative for estrogen receptor (ER), positive for progesterone receptor (PR), and positive for inhibin (Fig. 2).
The patient received two cycles of systemic combination chemotherapy with paclitaxel (175 mg/m2) and carboplatin (AUC 5) every 3 weeks. However, the masses in the abdomen and pelvic cavity increased, resulting in difficulty in voiding and defecation (Fig. 1C). Finally, we tried debulking surgery, despite the multiple disseminated disease status, for palliation. The debulking surgery failed because of peritoneal dissemination, with severe adhesion to the bowel and retroperitoneum; thus, she underwent a colostomy.
Subsequently, her performance status declined, but she did not want any further chemotherapy. After surgery, she underwent palliative radiotherapy (6,000 cGY/30 Fr) for the pelvic masses obstructing her urinary tract and bowel, and monthly hormonal therapy with a GnRH agonist (leuprorelin 3.75 mg IM). She tolerated this treatment without difficulty. One month after completing radiotherapy and after three GnRH agonist injections, a CT scan revealed that the multiple seeded masses, including those both within and beyond the radiotherapy field, had partially decreased (Fig. 1D). Her partial response was calculated as 36.5% decreased, using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria, for the target lesions in the abdominal cavity, excluding the pelvic mass. With the change in the tumors, her blood estradiol level decreased (Fig. 3). The partial response had been maintained for more than 8 months as of her last follow-up visit. Currently, the patient is doing well, with no discomfort or pain, and continues to receive hormonal therapy.
Granulosa cell tumors are uncommon, and thus experience in managing this tumor is limited. There is no standard approach to the management of relapsed GCTs, although a combination of approaches, including surgery followed by radiation or chemotherapy, has been associated with prolonged disease-free survival in some series (3, 4). Regarding chemotherapy as a treatment modality, platinum-based regimens have been the treatment of choice for the past decade and are the preferred option for treating widespread disease or disease that is suboptimally cytoreduced at the time of relapse.
There are limited data on the use of radiation therapy in the treatment of GCTs. Wolf et al. (4) showed a 43% response rate in patients with advanced stage or recurrent GCT who were treated with whole abdomen irradiation or pelvic irradiation. Some case reports describe the use of radiation treatment for isolated liver or bone recurrences (5, 6). However, there is no clear role for radiotherapy because of the lack of uniformity in staging and in treatment regimens.
In the present case, the outcome of chemotherapy was disappointing. The low metabolic rates of the masses recognized by PET-CT may have been the reason for the limited effect of chemotherapy. In addition, the irradiation of the whole abdomen or the pelvic cavity in this case was very challenging because of the large tumor size the disseminated status of the cancer. Thus, other treatment options were needed in this case.
GCT is a hormonally active tumor, and thus it seemed reasonable to assess whether hormonal agents might be active against these tumors. There have been several reports about the hormonal therapy of GCTs with GnRH agonists, aromatase inhibitors, or megesterol (7-11). Several mechanisms have been suggested for how hormone manipulation may inhibit tumor growth in GCT (11-14). These can be categorized as indirect action on tumors via suppression of gonadotropins or endogenous steroids and direct effects on the tumor via a local mechanism mediated by specific receptors in the GCT. A proportion of GCTs expresses receptors for follicle stimulating hormone (FSH), which has been shown to support the growth of GCTs in nude mice (15). Thus, hormonal therapies that can decrease gonadotropins may block the stimulatory effects on granulosa cells. GnRH agonists have previously been used in other hormonally regulated cancers, such as breast and prostate cancer (16, 17). Estrogen stimulates proliferation of granulosa cells by increasing the cells' responsiveness to FSH, thus, anti-estrogens may have anti-proliferative activity in GCT. In addition, progestins were suggested as chemopreventive agents by inducing apoptosis pathway involving transforming growth factor (TGF-α) in ovarian epithelium, a plausible local mechanism for inhibiting tumor growth (18). However, experience in treating GCT with hormonal agents is limited. Although several studies have reported the successful use of GnRH agonists (7, 8), negative results have also been reported (9). Furthermore, hormonal therapy has been attempted in cases of progressive GCTs that have failed to respond to chemotherapy and/or radiation. Based on the clinical course and performance status in our case, we considered hormonal treatment to be appropriate.
The hormonal activity of GCTs permits the use of a variety of tumor markers for postoperative surveillance. Estradiol was one of the first substances identified as being secreted by GCTs and is responsible for some of the clinical manifestations. However, there was no consistent correlation between the estradiol level and GCT progression in a previous report (19). In our case, the estradiol level appeared to correlate with the clinical course. Larger studies are needed to further examine the usefulness of estradiol as a marker for monitoring the status of GCT patients.
The natural course of GCT usually involves a very long history, and relapse can occur many years after the initial diagnosis. Owing to its long and indolent course and the low metabolic rate of the tumors, advanced or recurrent GCT requires treatment options beyond chemotherapy, surgery, and radiotherapy. Hormonal agents may provide another treatment option for advanced or recurrent GCT in those who are not candidates for surgery, chemotherapy, or radiotherapy.
Figures and Tables
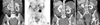 | Fig. 1Images before and after treatments. (A) A CT scan performed before systemic chemotherapy shows multiple metastatic masses in the abdomen and pelvis. (B) A PET scan performed before systemic chemotherapy shows multiple hypometabolic masses in the abdomen and pelvis. (C) A CT scan performed before radiotherapy and hormonal therapy shows multiple metastatic masses with increased size in the abdomen and pelvis. (D) A CT scan performed after radiotherapy and hormonal therapy shows a partial response to this therapy. The insert shows a radiotherapy planning radiography. |
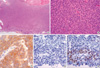 | Fig. 2Photomicrographs of recurrent granulosa cell tumor. Note the classic grooved nuclei, known as "coffee bean" nuclei, in the malignant granulosa cells (A: H&E, ×40; B: H&E, ×400) and the positive immunohistochemical staining for inhibin (C: ×400), progesterone receptor (E: ×400), and negative staining with estrogen receptor (D: ×400). |
References
1. Hartmann LC, Young RH, Podratz KC. Hoskins WJ, Perez CA, Young RC, editors. Ovarian sex cord-stromal tumors. Principles and practice of gynecologic oncology. 1993. 3rd ed. Lippincott Williams and Wilkins;1075–1093.
2. Miller BE, Barron BA, Dockter ME, Delmore JE, Silva EG, Gershenson DM. Parameters of differentiation and proliferation in adult granulosa cell tumors of the ovary. Cancer Detect Prev. 2001. 25:48–54.
3. Muntz HG, Goff BA, Fuller AF Jr. Recurrent ovarian granulosa cell tumor: role of combination chemotherapy with report of a long-term response to a cyclophosphamide, doxorubicin and cisplatin regimen. Eur J Gynaecol Oncol. 1990. 11:263–268.
4. Wolf JK, Mullen J, Eifel PJ, Burke TW, Levenback C, Gershenson DM. Radiation treatment of advanced or recurrent granulosa cell tumor of the ovary. Gynecol Oncol. 1999. 73:35–41.

5. Kumar PP, Good RR, Linder J. Complete response of granulosa cell tumor metastatic to liver after hepatic irradiation: a case report. Obstet Gynecol. 1986. 67:Suppl 3. 95S–98S.
6. Dubuc-Lissoir J, Berthiaume MJ, Boubez G, Van Nguyen T, Allaire G. Bone metastasis from granulosa cell tumor of the ovary. Gynecol Oncol. 2001. 83:400–404.
7. Fishman A, Kudelka AP, Tresukosol D, Edwards CL, Freedman RS, Kaplan AL, Girtanner RE, Kavanagh JJ. Leuprolide acetate for treating refractory or persistent ovarian granulosa cell tumor. J Reprod Med. 1996. 41:393–396.
8. Kauppila A, Bangah M, Burger H, Martikainen H. GnRH agonist analog therapy in advanced/recurrent granulosa cell tumors: further evidence of a role of inhibin in monitoring response to treatment. Gynecol Endocrinol. 1992. 6:271–274.

9. Maxwell GL, Soisson AP, Miles P. Failure of gonadotropin releasing hormone therapy in patients with metastatic ovarian sex cord stromal tumors. Oncology. 1994. 51:356–359.

10. Freeman SA, Modesitt SC. Anastrozole therapy in recurrent ovarian adult granulosa cell tumors: a report of 2 cases. Gynecol Oncol. 2006. 103:755–758.

11. Malik ST, Slevin ML. Medroxyprogesterone acetate (MPA) in advanced granulosa cell tumours of the ovary-- a new therapeutic approach? Br J Cancer. 1991. 63:410–411.
12. Liu J, Hyden-Granskog C, Voutilainen R. Gonadotrophins inhibit and activin induces expression of inhibin/activin beta(B) subunit mRNA in cultured human granulosa-luteal cells. Mol Hum Reprod. 2001. 7:319–323.

13. Lovell TM, Gladwell RT, Groome NP, Knight PG. Modulatory effects of gonadotrophins and insulin-like growth factor on the secretion of inhibin A and progesterone by granulosa cells from chicken preovulatory (F1-F3) follicles. Reproduction. 2002. 123:291–300.

14. Meyer JS, Rao BR, Valdes R Jr, Burstein R, Wasserman HC. Progesterone receptor in granulosa cell tumor. Gynecol Oncol. 1982. 13:252–257.

15. Davy M, Torjesen PA, Aakvaag A. Demonstration of the FSH receptor in a functioning granulosa cell tumour. The effect of gonadotrophin treatment on its viability following transplantation to nude mice. Acta Endocrinol (Copenh). 1977. 85:615–623.
16. Cook T, Sheridan WP. Development of GnRH antagonists for prostate cancer: new approaches to treatment. Oncologist. 2000. 5:162–168.

17. Robertson JF, Blamey RW. The use of gonadotrophin-releasing hormone (GnRH) agonists in early and advanced breast cancer in pre- and perimenopausal women. Eur J Cancer. 2003. 39:861–869.

18. Rodriquez G. New insights regarding pharmacologic approaches for ovarian cancer prevention. Hematol Oncol Clin North Am. 2003. 17:1007–1020.
19. Rey RA, Lhomme C, Marcillac I, Lahlou N, Duvillard P, Josso N, Bidart JM. Antimu_llerian hormone as a serum marker of granulosa cell tumors of the ovary: comparative study with serum alpha-inhibin and estradiol. Am J Obstet Gynecol. 1996. 174:958–965.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download