Abstract
Clostridium difficile (C. difficile) is a common causative agent of pseudomembranous colitis (PMC). C. difficile-associated diarrhea (CDAD) ranges from mild diarrhea to life threatening PMC. Recently, a highly virulent strain of C. difficile polymerase chain reaction ribotype 027 was found in North America, Europe, and Japan. A 52-yr-old woman with anti-tuberculosis medication and neurogenic bladder due to traffic accident experienced five episodes of C. difficile PMC after taking antibiotics for pneumonia along with septic shock and acute renal failure. She was readmitted to the intensive care unit and treated with oral vancomycin with refractory of oral metronidazole, inotropics and probiotics for over 60 days. C. difficile isolated both at the first and the last admission was identified as C. difficile ribotype 027 by ribotyping, toxinotyping, and tcdC gene sequencing, which turned out the same pathogen as the epidemic hypervirulent B1/NAP1 strain. This is the first case of C. difficile PCR ribotype 027 in Korea. After discharge, she was maintained on probiotics and rifaximin for 3 weeks. She had no relapse for 6 months.
Clostridium difficile (C. difficile) is the predominant causative agent of the nosocomial and antibiotic-associated diarrhea (1). The clinical presentation of C. difficile-associated disease (CDAD) varies from asymptomatic colonization to mild diarrhea to severe life-threatening pseudomembranous colitis (PMC) (1-4). Fulminant colitis develops in approximately 1 to 3% of the patients, leading to ileus, toxic megacolon, perforation, and death (1). PMC is known to develop after the antibiotic administration, whereas the antituberculosis agents are rarely associated with this disorder (1, 5).
In recent years, a high-leveled toxin-producing strain of C. difficile polymerase chain reaction (PCR) ribotype 027 has been isolated in North America, Europe (4), and Japan (6), which were associated with a more severe course, higher mortality, increased risk of relapse, and complication. This increased virulence is presumably associated with higher levels of toxin production in C. difficile PCR ribotype 027 strains (3, 4). This mechanism involves a partial deletion in the tcdD gene encoding a down-regulator of the expression of toxins A and B, as well as the production of a binary toxin, whose role in the pathogenesis is yet unknown (3, 4). We report the first isolation case of C. difficile PCR ribotype 027 from a patient with refractory and fulminant C. difficile PMC in Korea.
A 52-yr-old woman, taking oral vancomycin 125 mg daily for recurrent PMC, was readmitted with high fever and recurrent watery diarrhea in late April 2008. She had been discharged 22 days ago, after a prolonged stay in the intensive care unit (ICU) with C. difficile PMC secondary to the septic shock and acute renal failure.
She has had a traumatic paraplegia and neurogenic bladder for several years. At first admission, she had been treated for active pulmonary tuberculosis combined with pneumonia; rifampin (600 mg/day), isoniazid (300 mg/day), ethambutol (800 mg/day), pyrazinamide (1,500 mg/day), levofloxacin (750 mg/day), amikacin (750 mg/day) and azithromycin (500 mg/day) since December 5, 2007. Until pneumonic consolidation had improved; levofloxacin, amikacin, and azithromycin were used during 8, 5, and 3 days, respectively. She developed C. difficile PMC with septic shock on day 13 of anti-tuberculosis therapy, and then she had four documented episodes of C. difficile PMC (Fig. 1). C. difficile toxin A/B assay of the stool culture taken on admission day 3 gave positive result on C. difficile. On admission day 15, the sigmoidoscopic examination revealed scattered whitish to yellowish pseudomembranes with edematous hyperemic mucosa from the rectum to the descending colon (Fig. 2). Biopsy from the sigmoid colon revealed acute and chronic inflammation (Fig. 3). With a refractory of oral metronidazole for 8 days, she was treated with oral vancomycin and became well after 4 weeks. In spite of the oral vancomycin maintenance therapy, she was repeatedly readmitted with C. difficile PMC and required a stay in ICU due to the septic shock.
On this admission, her blood pressure was 60/30 mmHg, pulse rate 100/min, respiratory rate 20/min, and body temperature elevated to 38.4℃. Her lip and tongue were severely dehydrated, but her breathing sounds were normal on both lung fields. Her abdomen was soft and obese with direct tenderness in the whole abdomen area, and her bowel sounds were hyperactive. Laboratory data on admission were as follows: hemoglobin 10.0 g/dL, hematocrit 30.7%, white blood cell count 1,700/µL with 70.7% segmental neutrophil, platelet 162,000/µL, ESR 3 mm/hr, and CRP 32.29 mg/dL. Prothrombin and partial thromboplastin times were slightly prolonged to 12.8 (76%) and 30.7 sec, respectively. Liver function test results were: total protein 4.8 g/dL, albumin 2.5 g/dL, total bilirubin 0.4 mg/dL, direct bilirubin 0.2 mg/dL, ALT 12 IU/L, and AST 16 IU/L. Furthermore, BUN was 38 mg/ dL, creatinine 2.8 mg/dL, serum sodium 135 mEq/L, potassium 5.2 mEq/L, chloride 100 mEq/L, and EtCO2 18 mEq/L.
Despite vigorous hydration and inotropics, the septic shock was out of control. Intravenous immunoglobulin and meropenem were administered, and oral vancomycin (250 mg four times daily), with suspicious recurrent of the 5th C. difficile PMC, was prescribed to the patient. Anti-tuberculosis agents were discontinued due to an adequate course for 6 months. Diarrhea and fever was subsided after 5 days.
Blood and urine cultures were negative; thus, meropenem was discontinued. Stool specimens were anaerobically cultured in each episode, and all isolates were identified as C. difficile by Vitek ANI card (bioMérieux®Sa, Marcy l'Etoile, France). Stool specimens were positive for toxin A/B using VIDAS C. difficile CDAB (bioMérieux®Sa, Marcy l'Etoile, France). We characterized the C. difficile strain isolated at both the first and the last admissions. The Clinical and Laboratory Standards Institute agar dilution test revealed that both isolates were resistant to ampicillin (MIC=2 µg/mL), cefoxitin (MIC>128 µg/mL), cefotetan (MIC=128 µg/mL), clindamycin (MIC>128 µg/mL), imipenem (MIC=16 µg/mL) and moxifloxacin (MIC=16 µg/mL), and sensitive to piperacillin (MIC=16 µg/mL), vancomycin (MIC=2 µg/mL), and metronidazole (MIC=4 µg/mL).
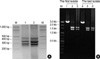
PCR ribotyping was performed using the method described by Stubbs et al. (7). Briefly, DNA was extracted by boiling bacteria suspension with 5% Chelex-100 (Sigma Co., St Louis, MO, USA). The supernatant (10 µL) of the DNA extract was added to a 40 µL PCR mixture containing 1.5 U of Taq polymerase (AmpliTaq Gold, Applied Biosystems, Foster City, CA, USA), 0.4 mM dNTP, 4.0 mM MgCl2, 10 mM Tris-HCl pH 8.30, 50 mM KCl (1X PCR Buffer II, Applied Biosystems) and 20 ρmol of each primer (5'-CTGGGGTGAAGTCGTAACAAGG-3'and 5'-GCGCCCTTTGTAGCTTGACC-3'). Reaction mixtures were subjected to 40 cycles of denaturation at 95℃ for 1 min, annealing at 55℃ for 1 min, and extension at 72℃ for 2 min and a last extension at 72℃ for 7min. A molecular size standard (100 bp; Invitrogen, Carlsbad, CA, U.S.A.) was run with an electrophoresis of amplification products, and analyzed with Molecular Analyst software (Bio-Rad Laboratory, Hercules, CA, U.S.A.). PCR ribotyping revealed that both the first and the last isolates were the same type as C. difficile ribotype 027 (Fig. 4A). Toxinotyping of C. difficile isolates was performed by the method of Rupnik et al. (8). Restriction patterns of this patient's isolates by B1 and A3 PCRs revealed toxinotype III (Fig. 4B). Sequencing of the tcdC gene of both the first and the last isolates using the primer pairs (5'-TCTCTACAGCTATCCCTGGT-3'and 5'-AAAAATGAGGGTAACGAATTT-3') (9) showed both 18-bp deletions and a single nucleotide deletion at position 117 (Fig. 5), identical to the tcdC-sc1 genotyping classified by Curry et al. (10).
She was placed on the vancomycin-tapering schedule of 125 mg at 3-day intervals for over 60 days and ultimately made a satisfactory recovery. The subsequent stool samples on the day 17 were negative for C. difficile toxin and culture. One day after completing the vancomycin treatment, she was discharged without complications. After discharge, she was maintained on probiotics and rifaximin for 3 weeks. She had no relapse for 6 months.
Since the first description of C. difficile by Hall and O'Toole in 1935, C. difficile has been recognized as the causative agent of the nosocomial diarrhea and the nearly exclusive cause of PMC in 1978 (11). At present, C. difficile is the cause of approximately 25% of all cases of antibiotic-associated diarrhea worldwide (12). The most common inducing agents have been known to be clindamycin and the broad-spectrum cephalosporin, but nearly all agents with an antibacterial spectrum may be responsible (1-4). On the other hand, anti-tuberculosis agents are rarely associated with this disorder (1, 5). Among these agents, rifampin is postulated to be the cause of PMC, because it has an antibiotic effect on a wide range of bacteria, whereas isoniazid and ethambutoal had little or no effect on the intestinal flora (13). In our case, the patient had taken not only antibiotic agents such as levofloxacin, amikacin, and azithromycin for several days, but also rifampin for 6 months. The latter might be the reason why she suffered from the recurrent fatal PMC five times for a long time.
The possible high risk factors for recurrent CDAD included patients with chronic renal failure, previous multiple episodes of CDAD, continued therapy using other antibiotics, community-acquired CDAD, high blood cell count (≥15×109/L), and those infected with certain strains of C. difficile (14, 15). Older age, a high leukocyte count, and renal failure at the first recurrence were also strongly associated with the complicated CDAD (16). In our case, major risk factors for recurrent PMC were previous multiple episodes of CDAD, long-term administration of anti-tuberculosis agent including rifampin, and, most of all, the highly virulent C. difficile.
Recommendations for treatment of CDAD are supportive care, withdrawal of the implicated antibiotics, and avoidance of antiperistaltic drugs and the use of oral metronidazole or oral vancomycin (1-3). Clinical resolution could be observed in almost 90% of the patients treated by oral metronidazole and vancomycin (2). Approximately 15% of the patients experienced relapse after initial therapy and required retreatment, sometimes with an extended, tapering regimen for 4- to 6-week period (1).
Recently, the epidemiology of C. difficile appears to be changing. Since 2002, outbreaks of severe CDAD associated with increased frequency and severity, as well as reduced responses to the metronidazole treatment have turned the attention onto a new strain (2, 6). The emergence of a new strain has changed the risk group, prognosis, and treatment strategies (3). This new strain is now known in North America as BI/NAP1 and in Europe as ribotype 027 (2, 17). It produces about 15 to 20 times the amount of toxins A and B due to the deletion of tcdC gene, which, under normal condition, negatively regulates the toxin production. Because toxins A and B are the primary virulence factors of C. difficile (2), the increased virulence could be due to the increased toxin production throughout the log phase of growth (4). In Quebec outbreaks, the rate of nosocomial disease was at least five times higher than the historical average (18). In addition, mortality rates rose up to 13.8%, and incidence on individuals aged 65 yr or more increased from 102 per 100,000 in 1991-1992 to 866 per 100,000 in 2003 (4).
Currently, the standardized treatment for the highly virulent C. difficile strain, which does not respond to oral metronidazole and vancomycin, is not available (19); thus colectomy is presently the considerable treatment choice (20). However, the mortality rate of this procedure ranges from 35 to 57% (3, 12). Various investigational trials, such as biotherapy, probiotics, stool implants, vaccination, and tapering and pulse-dosing of oral vancomycin, are being studied as potential treatments for severe CDAD (3, 12). Intravenous immunoglobulin may have beneficial effects on patients with refractory, recurrent or severe disease; however, no controlled data are available (19). In the present case, pulse-dosing of oral vancomycin, probiotics, and immunotherapy were used.
At present, it is not easy for physicians to confirm this highly virulent C. difficile strain. Because this strain is not universal, most laboratories are not equipped to perform PCR, which requires technical expertise, on a routine basis (21, 22). We suspected the presence of a highly virulent C. difficile strain, and thus the sample was referred to a research laboratory for detection the strain. PCR ribotyping appears to be the best method for initial detection, being reproducible, quick and easy to perform, and sufficiently discriminatory, it uses specific primers complementary to the 3' end of the 16S rRNA gene and to the 5' end of the 23S rRNA gene to amplify the variable-length intergenic spacer regions (23). Isolates from the patient were identified as the hypervirulent C. difficile 027 by clinical manifestations and molecular typing. To the best of our knowledge, this is the first case report of the C. difficile PCR ribotype 027 strain in Korea. Considering the virulence of this strain, it is important to be aware of the emergence of C. difficile in Korea to prevent the spreading of this strain. The emergence of C. difficile PCR ribotype 027 could be an implication of outbreaks associated with severe CDAD. Prevention is best accomplished by judicious use of antibiotics and infection control programs (24). In addition, C. difficile PCR ribotype 027 should be suspected, if there is an increase of prevalence or severity of CDAD. Early treatment is critical to the outcome of the patient and may reduce spreading of the organism by stopping diarrhea (25).
Figures and Tables
Fig. 1
Clinical course of the patient. Changes in serum CRP (gray line), diarrhea number (black bars), and medication per day are shown. HERZ, isoniazid 300 mg, ethambutol 800 mg, rifampin 600 mg, pyrazinamide 1,500 mg; Levo/Pip+TZ/AMK, levofloxacin, piperacillin, tazobatam, amikacin; IVIG, intravenous immunoglobulin; MDZ, metronidazole; Vanco, vancomycin; Adm, admission.

Fig. 2
Sigmoidoscopic examination revealed scattered whitish to yellowish pseudomembrane with edematous hyperemic mucosa from rectum to descending colon. (A) the first, (B) the second, (C) the third, and (D) the fourth admissions.

Fig. 3
A representative mucosal lesion with accompanying acute and chronic inflammation. Classic pseudomembranes were not present, but necroinflammatory exudates covered the mucosal ulcerations (H&E stain, ×200).
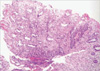
Fig. 4
PCR products and restriction patterns of C. difficile ribotype 027 isolates from the patient. (A) Identical PCR ribotype profiles obtained from both the first and the last isolates. Lanes: M, 100 bp DNA ladder; 1, the last isolate; 2, the first isolate. (B) Types of restriction patterns by B1 and A3 PCR showing toxinotype III. Lanes: M, DNA size marker; 1, 4, Acc I restriction patterns of B1 fragment; 2, 5, HincII restriction patterns of B1 fragment; 3, 6, EcoRI restriction patterns of A3 fragment.
References
1. Mylonakis E, Ryan ET, Calderwood SB. Clostridium difficile-associated diarrhea. Arch Intern Med. 2001. 161:525–533.
2. Aslam S, Hamill RJ, Musher DM. Treatment of Clostridium difficile-associated disease: old therapies and new strategies. Lancet Infect Dis. 2005. 5:549–557.
3. Bartlett JG. Narrative review: the new epidemic of Clostridium difficile-associated enteric disease. Ann Intern Med. 2006. 145:758–764.
4. Warny M, Pepin J, Fang A, Killgore G, Thompson A, Brazier J, Frost E, McDonald LC. Toxin production by an emerging strain of Clostridium difficile associated with outbreaks of severe disease in North America and Europe. Lancet. 2005. 366:1079–1084.
5. Jung SW, Jeon SW, Do BH, Kim SG, Ha SS, Cho CM, Tak WY, Kweon YO, Kim SK, Choi YH, Cha SI. Clinical aspects of rifampicin-associated pseudomembranous colitis. J Clin Gastroenterol. 2007. 41:38–40.

6. Kato H, Ito Y, van den Berg RJ, Kuijper EJ, Arakawa Y. First isolation of Clostridium difficile 027 in Japan. Euro Surveill. 2007. 12:E070111.3.
7. Stubbs SL, Brazier JS, O'Neill GL, Duerden BI. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J Clin Microbiol. 1999. 37:461–463.
8. Rupnik M, Avesani V, Janc M, von Eichel-Streiber C, Delmée M. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J Clin Microbiol. 1998. 36:2240–2247.
9. Spigaglia P, Mastrantonio P. Comparative analysis of Clostridium difficile clinical isolates belonging to different genetic lineages and time periods. J Med Microbiol. 2004. 53:1129–1136.
10. Curry SR, Marsh JW, Muto CA, O'Leary MM, Pasculle AW, Harrison LH. tcdC genotypes associated with Severe TcdC truncation in an epidemic clone and other strains of Clostridium difficile. J Clin Microbiol. 2007. 45:215–221.
11. Bartlett JG, Chang TW, Gurwith M, Gorbach SL, Onderdonk AB. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978. 298:531–534.

13. Mandelll GL, Petri WA Jr. Hardman JG, Limbird LE, editors. Antimicrobial agents (continued). Goldman and Gilman's the pharmacological basis of therapeutics. 1996. 9th edition. New York: McGraw-Hill;1155–1174.
14. Fekety R, McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, Mulligan ME. Recurrent Clostridium difficile diarrhea: characteristics of risk factors for patients in a prospective, randomized, double-blinded trial. Clin Infect Dis. 1997. 24:324–333.
15. Do AN, Fridkin SK, Yechouron A, Banerjee SN, Killgore GE, Bourgault AM, Jolivet M, Jarvis WR. Risk factors for early recurrent Clostridium difficile-associated diarrhea. Clin Infect Dis. 1998. 26:954–959.
16. Pepin J, Routhier S, Gagnon S, Brazeau I. Management and outcomes of a first recurrence of Clostridium difficile-associated disease in Quebec, Canada. Clin Infect Dis. 2006. 42:758–764.
17. You DM, Franzos MA, Holman RP. Successful treatment of fulminant Clostridium difficile infection with fecal bacteriotherapy. Ann Intern Med. 2008. 148:632–633.
18. Pepin J, Valiquette L, Cossette B. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. CMAJ. 2005. 173:1037–1042.
19. Gerding DN, Muto CA, Owens RC Jr. Treatment of Clostridium difficile infection. Clin Infect Dis. 2008. 46:Suppl 1. S32–S42.
20. Hermsen JL, Dobrescu C, Kudsk KA. Clostridium difficile infection: a surgical disease in evolution. J Gastrointest Surg. 2008. 12:1512–1517.
21. Kato N, Ou CY, Kato H, Bartley SL, Luo CC, Killgore GE, Ueno K. Detection of toxigenic Clostridium difficile in stool specimens by the polymerase chain reaction. J Infect Dis. 1993. 167:455–458.
22. Karasawa T, Nojiri T, Hayashi Y, Maegawa T, Yamakawa K, Wang XM, Nakamura S. Laboratory diagnosis of toxigenic Clostridium difficile by polymerase chain reaction: presence of toxin genes and their stable expression in toxigenic isolates from Japanese individuals. J Gastroenterol. 1999. 34:41–45.
23. Bidet P, Lalande V, Salauze B, Burghoffer B, Avesani V, Delmee M, Rossier A, Barbut F, Petit JC. Comparison of PCR-ribotyping, arbitrary primed PCR, and pulsed-filed gel electrophoresis for typing Clostridium difficile. J Clin Microbiol. 2000. 38:2484–2487.
24. Gerding DN, Muto CA, Owens RC Jr. Measures to control and prevent Clostridium difficile infection. Clin Infect Dis. 2008. 46:Suppl 1. S43–S49.
25. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis. 2008. 46:Suppl 1. S12–S18.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download