Abstract
EC-18 (monoacetyldiacylglyceride) stimulates T cell production of IL-2, IL-4, IL-12, IFN-γ, and GM-CSF in vitro. To study the effects of these cytokines stimulated by EC-18 on cancer cells, we applied hamster biliary cancer model, a difficult cancer to treat. Cancer (KIGB-5) cells were given intravenously to produce hematogenous metastatic lung lesions which were treated with EC-18 at 10, 25, and 50 mg/kg/day respectively. The fourth group was untreated control. At 4th, 8th, and 12th week the lungs were examined. EC-18 treated groups showed only a few microscopic lung lesions and no evidence of metastatic lesion with highest dose whereas widespread gross lung lesions were observed in untreated control. To investigate whether the anti-tumor effect of EC-18 is associated with suppression of tumor cell Toll-like receptor 4 (TLR-4) expression in addition to stimulation of the immune cells, KIGB-5 cells were exposed to LPS with or without EC-18. TLR-4 mRNA and protein expression, measured by reverse transcriptase PCR (RT-PCR), real-time quantitative PCR and western blot analysis, showed suppression of TLR-4 expression in KIGB-5 cells treated with EC-18 compared with control. In conclusion, EC-18 has a significant anti-tumor effect in this experimental model of biliary cancer suggesting potential for clinical application to this difficult cancer.
Cholangiocarcinoma is a malignancy arising from the epithelial cells of the bile duct or gallbladder. Although it occurs with greater frequency in Asian countries, its incidence has been steadily increased in U.S.A. and Europe over the past three decades (1, 2). Surgery still remains the only curative treatment, but many of these tumors are not resectable at the time of diagnosis. Chemotherapy and radiation therapy have been of limited value (1). A variety of biological response modifiers, including cytokines and bacterial products, have been employed in immunochemotherapy of various cancers. High dose Interleukin (IL)-2 has been tested against various malignant tumors, both in experimental models and in clinical studies, but its effectiveness has been hampered by undesirable side effects (3).
EC-18 is a monoacetyldiglyceride (EC-18 specifically, 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol) that occurs naturally in various seed oils, in bovine udder and in milk fat, and has been isolated from the antlers of sika deer (Cervus nippon TEMMENICK); these antlers have been used as a tonics in oriental medicine. We have shown that chemically synthesized EC-18 can stimulate the proliferation of hematopoietic stem cells, bone marrow stromal cells (4, 5), and immune system cells, including T and B lymphocytes, dendritic cells (DCs) and macrophages, both in vivo and in vitro. Using the Syrian golden hamster model of biliary cancer, we observed previously that IL-2 encoded bone marrow stromal cells (ad/hIL-2 BMSC) suppressed metastatic tumor (6). EC-18 was an effective agent as in inhibiting the growth of metastatic biliary cancer.
Toll-like receptors (TLRs), especially TLR-4, has been shown to be important in both innate and adaptive immunity to various cancers. Activation of TLR-4 in tumor cells was found to stimulate cancer cell proliferation, whereas inhibition of TLR-4 signaling retarded tumor growth and prolonged the survival of tumor bearing mice (7). EC-18 has been shown to have inhibitory effects on TLR-4 expression of experimental biliary cancer cells (KIGB-5).
We report here the use of a novel agent of EC-18 as metastasis inhibiting agent in hamster biliary cancer model (KIGB-5). This suppression of metastasis is likely through stimultion of immune cells (T & B cells, DC & macrophages) in association with suppression of tumor cell TLR-4 expression.
Female Syrian golden hamsters (6-8 weeks old) were purchased from Harlan (Indianapolis, IN, U.S.A.) and housed in the specific pathogen free unit (temperature, 22±2℃; humidity 60±4%, 12 hr light/dark cycle) at the Animal Resource Center at the Asan Institute for Life Science. Water was provided ad libitum. All animals used in this experiment were cared for and used humanely according to the NIH Principles of Laboratory Animal Care (U.S. NIH publication No. 85-23, Revised 1985) and the guidelines for animal experiments of the Asan Institute for Life Sciences and Technology. The Syrian golden hamster cholangiocarcinoma cell line, KIGB-5, was cultured in complete RPMI-1640 medium (GIBCO BRL, Grand Island, NY, U.S.A.) supplemented with 10% fetal bovine serum (FBS) (GIBCO BRL).
EC-18 was synthesized by the patented process (8) (Korean patent appl. 10-2005-0065792 [2005.7.20]) from 1-palmitoylglycerol as a starting material.
The purity of EC-18 in this paper was determined above 99% using HPLC method (Instrument: Younlin Solvent Delivery Pump M930, Younlin UV Absorbance Detector M720; Column: YMC-pack ODS-A A-303 250×4.6 mm I.D., S-5M, 12 nm; Detector: 205 nm; Mobile phase: Isopropanol: Acetonitrile=45:55; Temperature: 25℃, Flow rate: 1 mL/min).
Splenocytes were collected by flushing the spleen of mice and single cell suspensions were obtained by repeated aspiration and flushing. Cell preparation was suspended in Iscove's modified Dulbecco's medium (IMDM, GIBCO) supplemented with 10% FBS, with 5×104 viable cells per well cultured for 5 days with EC-18 0.01, 0.1, and 1 µg/mL or rm IL-2 10, 20 ng/mL, each in triplicates. On day 4, 1 µCi 3H-thymidine was added to each well, and the cells were cultured for an additional 18 hr. On day 5, the cells were harvested and 3H-thymidine incorporation was measured. Stimulation index (SI) was calculated as CPM in sample/CPM in control.
T4 and T8 cells were purified using magnetic beads, goat anti-mouse CD4 and CD8, respectively (MACS bead, Miltenvi Biotec, Bergich Gladbach, Germany), and anti-goat IgG. Each T cell preparation was suspended in Iscove's modified Dulbecco's medium (IMDM, GIBCO) supplemented with 10% FBS, with 2×106 viable cells per well cultured for 5 days with EC-18 0.01, 0.1, and 1 µg/mL or rm IL-2 10, 20 ng/mL. The number of IL-2 producing cells was counted using rm IL-2 Elispot system kit (AID, Strasburg, Germany) according to the manufacturer's instruction.
Anti-CD3 monoclonal antibody (Pharmingen, Hamburg, Germany) was coated onto 96-well plates. T cells were seeded at 2×105 viable T cells per well and cultured with 5 µg/mL anti-CD28 MoAb (Pharmingen). EC-18 was added to the culture media at 0.1 or 1.0 µg/mL, with no EC-18 as control, and the culture media were harvested on day 5. The supernatants were obtained by centrifugation, and the concentrations of secreted cytokines were quantified using a Bio-plex kit (Bio-rad, Washington, DC, U.S.A.) according to the manufacturer's instruction.
Hamster was treated with EC-18 50 mg/kg/day orally for 2 weeks and then splenocytes were harvested from control and EC-18 treated group. Measurement of Calcium ion was performed at day 1. An increase in [Ca2+]i was directly measured in splenocytes by change in the fluorescence intensity of fluo-3 AM (Molecular Probe, Inc., Eugene, OR, U.S.A.) loaded cells. Cells (5×106/mL) were incubated with HBSS buffer (Hank's balanced salt solution without Ca2+, Mg2+) containing 4 µM Fluo-3AM and 0.02% Pluonic F-127 (Molecular Probe, Inc.) in DMSO for 30 min at 37℃, Cells were then washed twice with HBSS buffer and resuspended with 5 mM Calcium chloride containing HBSS buffer. We followed changes in [Ca2+]i for a period of 0-120 sec after stimulation of splenocytes with 5 mM Ionomycin. Analysis was performed on the flowcytometry equipped with the 525 nm.
Hamsters were injected in their livers with 1×105 KIGB-5 cells and treated with 50 mg/kg/day EC-18 or PBS (control). After 8 weeks, when liver tumors had developed, splenocytes were obtained from tumor bearing hamsters. Cytotoxic activity was measured by incubating splenocytes with 2×104 target KIGB-5 cells, which had been labeled with 51Cr for 2 hr and washed three times, at a splenocyte: target cell ratio of 25:1, in 96-well round bottom plates in triplicate. After incubation for 4 hr at 37℃, the supernatants were harvested, and the amount of 51Cr released was measured with a Packard Parias gamma spectrometer (Packard Instrument, Meridian, CT, U.S.A.). Maximum and spontaneous releases were determined and the percentage of specific 51Cr release was calculated as: Percent specific cytotoxicity=(Specific release-spontaneous release)÷(maximum release-spontaneous release)×100.
KIGB-5 cells were exposed in vitro with 10 µg/mL lipopolysaccharide (LPS) and/or 1 µg/mL EC-18 and the cells were harvested. Total RNA was isolated using RNeasy kits (Qiagen) and reverse transcribed with Superscript I (Life Technologies) and random hexamers. The polymerase chain reaction was performed using Taq DNA polymerase (Takara, Tokyo, Japan) and primers specific for hamster TLR-4 (forward: 5'-GCAGGAACACCTACCTAGA-3'; reverse, 5'-GCTTGAT ACAGTAGGAGCTG-3'; product size, 402 bp) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; forward, 5'-GTGGAGATTGTTGCCATCAACG-3'; reverse, 5'-CAGTG GATGCAGGGATGATGTTCTG-3'; product size, 507 bp) as the endogenous control. Amplification conditions consisted of 30 cycles of denaturation at 95℃ for 30 sec, annealing at 52℃ for 30 sec, and extension at 72℃ for 40 sec. The PCR products were electrophoresed on 2% agarose gels, which were stained with ethidium bromide for visualization.
Real-time quantitative PCR was performed in triplicate in 384-well plates; each 20 µL reaction consisted of 10 µL of SYBR Green Master Mix (Applied Biosystems Foster City, CA, U.S.A.), 0.8 µL of 10 pM/µL forward and reverses primers of hamster TLR-4 and GAPDH. 50℃ for 2 min and 95℃ for 10 min followed by 40 cycles of 95℃ for 30 sec and 60℃ for 30 sec, 72℃ for 30 sec. The sequences of the primers designed to span within hamster TLR-4 (forward primer: GCATGGCCTTCCGTGTTCCTA, reverse primer: CTTCAGTGGGCCCTCAGATGC). The real-time PCR analysis was performed on a Prism 7900 Sequence Detection System (Applied Biosystems).
Western blot analysis was conducted as previously described (9). Primary antibody to rabbit serum anti-mouse Toll-like receptor (TLR-4, 1:1,000 dilution, Ebioscience, San Diego, CA, U.S.A.) was applied overnight at 4℃ Secondary antibody conjugated to horseradish peroxidase was applied the following day, followed by detection using enhanced chemiluminescence (ECL) (Amersham Biosciences, Little Chalfort, U.K.).
The same blots were also probed with a monoclonal antibody β-actin (1:500, Biolegend, San Diego, CA, U.S.A.) to verify equal protein loading in all lanes. Band densities were quantified by means of a Bio-Rad Versa Doc Imaging System (Bio-Rad, Washington, DC, U.S.A.).
Each of 20 hamsters was injected in the femoral vein with 5×105 KIGB-5 cells in 100 µL of serum free RPMI 1640. The hamsters were divided into 4 groups of 5 each: The control group was treated only with RPMI. The remaining 3 groups were administered 10, 25, and 50 mg/kg body weight/day of oral EC-18, 2 weeks on and 1 week off for 12 weeks. On the 4th week, one animal from each group was sacrificed and two animals from each group were sacrificed at the 8th and 12th week for pathological examination. And their livers and lungs were removed, fixed in formalin, stained with hematoxylin and eosin, and examined by light microscopy.
The effects of EC-18 on T-cell proliferation were tested by 3H-thymidine uptake. Cells treated with EC-18 1 µg/mL showed a 2.13 fold increase in 3H-thymidine uptake compared with control untreated cell (P<0.005). EC-18 0.1 µg/mL treated cells were also increased 2.07 fold compared with control cells (P<0.05). Cells treated with IL-2 20 ng/mL showed a 2.02 fold increase compare with control untreated cells (P<0.05). Cells treated with 0.01, 0.1, and 1 µg/mL of EC-18 were stimulated in dose dependent manner (Table1).
Following 5 days in culture with EC-18 1 µg/mL, the number of IL-2 producing cells was counted AID Elispot Reader System. The number of IL-2 producing T4 cells was increased 1.52-fold with EC-18 treatment which was statistically significant (P<0.05). The difference in the number of IL-2 producing T8 cells was not statistically significant (P<0.26) (Fig. 1).
Cytokine secretion was measured by using Bio-plex. As shown in Fig. 2, the secretions of IL-2 and IL-4 were much higher in EC-18 treated group than in the control (P<0.05 and P<0.005 respectively). The secretions of IL-12, GM-CSF and IFN-γ were also increased in EC-18 treated group compared with control (P<0.05).
EC-18 treated splenocytes showed 2.9 times increased [Ca2+]i influx than control (Fig. 3).
Cytolytic activity of T cells was measured by 51Cr release from pre-labeled tumor cells. At a T-cell to target cell ratio of 100:1, specific cytolysis was 19.5±0.37% in EC-18 treated cells compared with 13.7±0.19% in untreated control cells (P<0.05), and at a 25:1 ratio, it was 16.6±0.28% in EC-18 treated cells compared with 5.1±0.10% in untreated control cells (P<0.005).
The expression of TLR-4 mRNA was assayed by reverse transcriptase PCR (RT-PCR) and real-time quantitative PCR on KIGB-5 cells which were untreated control, treated with LPS (10 µg/mL) only, EC-18 (1 µg/mL) only and LPS with EC-18 treatment group. Treatment of KIGB-5 cells with EC-18 (1 µg/mL) reduced the expression of TLR-4 mRNA when compared with untreated control and LPS (10 µg/mL) treated cells (Table 2, Fig. 4).
We examined the levels of TLR-4 in KIGB-5 cells which were untreated control, treated with LPS (10 µg/mL) only, EC-18 (1 µg/mL) only and LPS with EC-18 treatment group by western blot analysis. Treatment of KIGB-5 cells with EC-18 (1 µg/mL) reduced the level of TLR-4 protein when compared with untreated control and LPS (10 µg/mL) treated cells (Fig. 5).
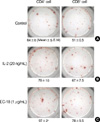
At the 4th week, all experimental groups showed no evidence of disease in the lungs, except a single microscopic lesion in control group and EC-18 10 mg/kg/day treated group. At the 8th week, animals in the control group showed widespread metastases in the both lungs. Group treated with 10, 25, and 50 mg/kg/day EC-18 showed no gross tumor, but microscopic examination revealed 2 microscopic lesions in 10 mg/kg/day group, 1 microscopic lesion in 25 mg/kg/day group and no microscopic lesions in 50 mg/kg/day group in the lungs (Table 3, Fig. 6).
At the 12th week, both animals in the control group had numerous conglomerated lesions in the lungs. In contrast, animals in EC-18 treated groups had no evidence of gross tumors. Animals treated with EC-18 (50 mg/kg/day) showed no evidence of metastatic lesions throughout the experimental period (Table 3, Fig. 7).
We previously reported that the water extracts from deer antler, Cervus nippon, had a stimulating effects on hematopoiesis in vitro and in vivo (4, 5), and we subsequently demonstrated that monoacetyldiglyceride (EC-18) was the component with the most potent stimulatory activity on the bone marrow stem cells, bone marrow stromal cells and immune cells.
In this paper, we showed that EC-18 has a potent anti-tumor activity in experizmental hematogenous metastatic biliary cancer in Syrian golden hamsters. We found that EC-18 stimulated the activity of CD4+, CD8+ cells and macrophages.
EC-18 treated CD4+ and CD8+ cells in vitro stimulate the production of cytokines; IL-2, IL-4, IL-12, IFN-γ, and GMCSF (Fig. 2). These cytokines enhance the cytolytic activity of NK cells and LAK-like lymphocytes (10-12), and tumor suppressor function of immune system is critically dependent on the action of IFN-γ and IL-12 (13, 14). The increased secretion of these cytokines may inhibit tumor growth and metastasis in this experiment.
In an attempt to understand the mechanism of action of EC-18 on the immune cells, we studied Ca2+ influx into lymphocytes. EC-18 treatment increases Ca2+ influx into lymphocytes (Fig. 3). Ca2+ is an essential component of signal transduction in lymphocytes (15, 16). We speculate that the proliferation of T cells, its differentiation to effector T cells, and the secretion of various cytokines we observed are mediated by the increase in the cytosolic (Ca2+) through canonical transient receptor potential channels (TRPC) activated by phospholipase C (PLC) coupled receptor. PLC in turn generates DAG by phosphoinositide cascade, and DAG activates protein kinase C (PKC) which modulates expressions of relevant genes (17, 19).
EC-18 has been shown to stimulate Ca2+ influx into rat pancreatic acinar cells (18). Long-chain diacylglycerols are, however, known to be impermeable to cells (19) and metabolically stable in the extracellular fluid. Therefore it is likely that the action of EC-18 on T cells is mediated through its interaction with the lymphocyte cell membrane. The exact mechanism of action of EC-18 needs to be elucidated.
Previously we reported that IL-2 gene-encoded stromal cells inhibited the growth and metastasis of cholangiocarcinoma (6). This immuno-cell therapy with ad/hIL-2 encoded stromal cells could be a promising therapeutic alternative as adoptive immunocell therapy for biliary cancers (6).
We tested the effects of EC-18 on Toll-like receptor 4 (TLR-4) mRNA and protein expression in KIGB-5 biliary cancer cells. EC-18 inhibited TLR-4 mRNA and protein expression in KIGB-5 cells.
TLR-4 is transmembrane proteins and represents newly recognized family of vertebrate pattern recognition receptors in innate immune system. Engagement of TLR-4 by one of its ligand triggers an intracellular signaling cascade that includes activation of latent cytoplasmic transcription factor NF-κB with it's translocation to the nucleus and activation of MAP kinase (20). LPS derived from Gram negative bacteria (endotoxin) is a ligand of TLR-4. Bacterial LPS-bound TLR-4 strongly stimulates innate immune responses that enhance killing of bacteria, but it may also cause significant pathological changes in the host. TLR-4 activation in tumor enhances immunosuppression in vitro, and escape of tumor cells from NK cell attack is also TLR-4 dependent (20). TLR-4 thus has dual effects on the immune system of tumor bearing animals. Toll-like receptors may initially play a critical role in both innate and adaptive immune response and an important role in immunity against various cancers. Activation of TLR-4 in tumor cells stimulates proliferation of cancer cells, while the blockade of TLR-4 signaling retards tumor growth and prolongs the survival of tumor bearing mice. Taken together, these findings indicate that TLR signaling induces a cascade that can lead to tumor evasion of immune surveillance.
We speculate that the observed inhibition of TLR-4 by EC-18 may have contributed to the retardation of tumor growth and metastasis in tumor bearing hamsters. These findings suggest that EC-18 may have positive therapeutic potentials in the treatment of biliary cancer.
Figures and Tables
 | Fig. 1IL-2 secretion by 1-palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (EC-18) treated T-cells. Brown spots indicate IL-2 producing cells. Data are expressed as mean±S.E.M. *P<0.05 compared with the control group. |
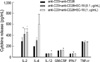 | Fig. 2Cytokine secretion by EC-18 treated T cells compared with Anti-CD3+Anti-CD28 treated control (IL-2, IL-4, IL-12[p70], GM-CSF, IFN-γ and TNF-α).
*P<0.05, †P<0.005 compared with the control group.
|
 | Fig. 3Effect of EC-18 on [Ca2+]i of mouse lymphocyte after Ionomycin exposure. Change in [Ca2+]i was compared with EC-18 treated group and control group for a period of 0-120 sec after exposure of Ca2+ mobilizing agent (5 mM Ionomyicin). |
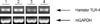 | Fig. 4Expression of TLR-4 in KIGB-5 cells by RT-PCR. EC-18 inhibits TLR-4 expression by RT-PCR in KIGB-5 cells. Line 1: Control: KIGB-5 were exposed medium alone, 2: LPS: KIGB-5 were exposed LPS (10 µg/mL), 3: EC-18: KIGB-5 were exposed EC-18 (1 µg/mL), 4: LPS + EC-18: KIGB-5 were exposed LPS and EC-18. |
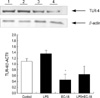 | Fig. 5Expression of TLR-4 in KIGB-5 cells by western blot analysis. EC-18 (1 µg/mL) treated cells reduced the level of TLR-4 protein when compared with untreated control and LPS (10 µg/mL) treated cells. Lane 1, Control: KIGB-5 were exposed medium alone; 2, LPS: KIGB-5 were exposed LPS (10 µg/mL); 3, EC-18: KIGB-5 were exposed EC-18 (1 µg/mL); 4, LPS+EC-18: KIGB-5 were exposed LPS and EC-18. Band densities were quantified by means of a Bio-Rad Versa Doc Imaging System. *P<0.05 compared with the control group (n=3). |
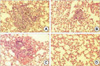 | Fig. 6Microscopic findings (H&E, ×100) of the lungs at 8 week after injection of KIGB-5 cells. (A) Control hamsters, (B) hamsters treated with EC-18 10 mg/kg/day, (C) EC-18 25 mg/kg/day, (D) EC-18 50 mg/kg/day. Control group showed multiple metastatic lesions. Hamster groups treated with EC-18 10, 25 mg/kg/day in respect showed metastatic lesions, except EC-18 50 mg/kg/day treated group which showed no evidence of the lesion. |
 | Fig. 7Gross pathological and microscopic findings (H&E, ×100) of the lungs at 12 weeks after injection of KIGB-5 cells. (A, a) Control hamsters, (B, b) hamsters treated with EC-18 10 mg/kg/day, (C, c) EC-18 25 mg/kg/day, (D, d) EC-18 50 mg/kg/day. Control group showed multiple metastatic lesions whereas, EC-18 10, 25, and 50 mg/kg/day treated group showed no evidence of the lesion. |
Table 2
TLR-4 real-time quantitative PCR analysis in KIGB-5 cells (biliary cancer cell line)

R2 of TLR-4: 0.9754, R2 of GAPDH: 0.9959.
Control: KIGB-5 cells were exposed medium alone. LPS: KIGB-5 cells were exposed LPS (10 µg/mL). EC-18: KIGB-5 cells were exposed EC-18 (1 µg/mL). LPS+EC-18: KIGB-5 cells were exposed LPS (10 µg/mL) and EC-18 (1 µg/mL).
Data are expressed as mean±S.E.M of triplicate of reactions.
*P<0.005 compared with the LPS and EC-18 or LPS+ EC-18.
ACKNOWLEDGMENT
KIGB-5 cell line was kindly provided by Dr. Yoshitsugu Tajima of Nagasaki University, Nagasaki, Japan.
References
3. Rosenberg SA, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of 283 consecutive patients with metastatic melanoma or renal cancer using high dose bolus interleukin-2. JAMA. 1994. 271:907–913.
4. Yang HO, Kim SH, Cho SH, Kim MG, Seo JY, Park JS, Jhon GJ, Han SY. Purification and structural determination of hematopoietic stem cell-stimulating monoacetyldiglycerides from Cervus nippon (deer antler). Chem Pharm Bull. 2004. 52:874–878.

5. Yang HO, Park JS, Cho SH, Yoon JY, Kim MG, Jhon GJ, Han SY, Kim SH. Stimulatory effects of monoacetyldiglycerides on hematopoiesis. Biol Pharm Bull. 2004. 27:1121–1125.

6. Kim MH, Lee SS, Lee SK, Lee SG, Suh CW, Gong GY, Park JS, Kim YH, Kim SH. Interleukin-2 gene-encoded stromal cells inhibit the growth of metastatic cholangiocarcinomas. World J Gastroenterol. 2006. 12:1889–1894.

7. Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, Mayer L, Unkeless JC, Xiong H. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005. 65:5009–5014.

8. Korean patent application. 10-2005-0065792. 2005. 07. 20.
9. Dimaio TA, Wang S, Huang Q, Scheef EA, Sorenson CM, Sheibani N. Attenuation of retinal vascular development and neovascularization in PECAM-1-deficient mice. DEV BIO. 2008. 315:72–88.

10. Hillman GG, Younes E, Visscher D, AIi E, Lam JS, Montecillo E, Pontes JE, Haas GP, Puri RK. Systemic treatment with interleukin-4 induces regression of pulmonary metastases in murine renal cell carcinoma model. Cell Immunol. 1995. 160:257–263.
11. Peace DJ, Kern DE, Schultz KR, Greenberg PD, Cheever MA. IL-4-induced lymphokine-activated killer cells. Lytic activity is mediated by phenotypically distinct natural killer-like and T cell-like large granular lymphocytes. J Immunol. 1988. 140:3679–3685.
12. Gambacorti-Passerini C, Rivoltini L, Supino R, Rodolfo M, Radrizzani M, Fossati G, Parmiani G. Susceptibility of chemoresistant murine and human tumor cells to lysis by interleukin-2 activated lymphocytes. Cancer Res. 1988. 48:2372–2376.
13. Shankaran V, Ikeda H, Bruce AT, White JM, Swanson PE, Old LJ, Schreiber RD. IFN-gamma and lymphocytes prevent primary tumor development and shape tumor immunogenicity. Nature. 2001. 410:1107–1111.
14. Egilmez NK, Hess SD, Chen FA, Takita H, Conway TF, Bankert RB. Human CD4+ effector T cells mediate indirect interleukin-12 and interferon-γ-dependent suppression of autologous HLA-negative lung tumor xenografts in severe combined immunodeficient mice. Cancer Res. 2002. 62:2611–2617.
15. Chuang M, Lee MW, Zhao D, Severson DL. Metabolism of a long chain diacylglycerol by permeabilized A10 smooth muscle cells. Am J Physiol. 1993. 265:C927–C933.
16. Forcic D, Mazuran R. Modulation of [Ca2+]i in freshly isolated mouse lymphocytes with in vivo priming. Immunol Lett. 1999. 67:23–30.
17. Randriamampita C, Trautmann A. Ca2+ signals and T-lymphocytes; "New mechanisms and functions in Ca2+ signaling". Biol Cell. 2004. 96:69–78.
18. Han SY, Cho SH, Kim SY, Seo JT, Moon SJ, Jhon GJ. Monoacetyl-diglycerides as new Ca2+ mobilizing agents in rat pancreatic acinar cells. Bioorg Med Chem Lett. 1999. 9:59–64.
19. Goni FM, Alonso A. Structure and functional properties of diacylglycerols in membranes. Prog Lipid Res. 1999. 38:1–48.
20. Okamoto M, Sato M. Toll-like receptor signaling in anti-cancer immunity. J Med Invest. 2003. 50:9–24.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download