Abstract
Elevated serum levels of interleukin-10 (IL-10) have been reported in patients with Kawasaki disease (KD). IL-10 reduces the inflammatory actions of macrophages and T cells and it may play a significant role in the regulation of inflammatory vascular damage associated with systemic vasculitis. The aim of this study was to examine whether -592 IL-10 promoter polymorphism is a susceptibility or severity marker of KD in Chinese patients in Taiwan. The study included 105 KD patients and 100 normal controls. Genotype and allelic frequencies for the IL-10 gene polymorphism in both groups were compared. There were no significant between-group differences in the genotype distribution of IL-10 A-592C gene polymorphism (P=0.08). However, the frequency of the -592*A allele was significantly increased in the patients with KD compared with controls (71.9% vs. 61.0%, P=0.019). The odds ratio for developing KD in individuals with IL-10-592*A allele was 1.64 (95% confidence interval, 1.06-2.52) compared to individuals with the IL-10-592*C allele. No significant difference was observed in the genotype and allelic frequencies for the IL-10 A-592C polymorphism between patients with and without coronary artery lesions. The IL-10-592*A allele may be involved in the development of KD in Taiwanese children.
Kawasaki disease (KD) is an acute systemic vasculitis that predominantly affects infants and young children. Coronary artery lesions (CALs) develop in 20-25% of the patients, which makes KD the leading cause of acquired heart disease in children. The cause of this disease is unknown but generally believed to be an infectious agent (1). Many reports have noted an abnormal immune response in the acute phase of this disease and increase in serum cytokines, such as interleukin (IL)-1β, IL-2, IL-4, IL-6, IL-8, interferon (INF)-γ, and tumor necrosis factor (TNF)-α(2-5). These cytokines may mediate endothelial damage and lead to coronary artery disease while also producing the systemic signs of inflammation that characterize the disease (6). Previous reports have shown blood IL-10 levels were also elevated during the acute phase of KD (5, 7, 8). The elevated IL-10 levels decreased immediately after intravenous immune globulin (IVIG) administration, and the decrease was coincident with rapid improvement in inflammatory symptoms (8). However, the significance of increased IL-10 levels observed in KD is not fully understood. IL-10 is a major immunoregulatory cytokine and has a number of immunomodulating effects on the immune system. It is produced by many cell types, including T lymphocytes, B lymphocytes, monocytes, and macrophages (9). IL-10 suppresses type 1 T helper lymphocytes by decreasing IL-2 and INF-γ production (10, 11). It also inhibits certain functions of activated macrophages by downregulating the expression of MHC class II antigens, and co-stimulatory molecules (12), and by inhibiting production of proinflammatory cytokines such as TNF-α, IL-1, IL-6, IL-8, and IL-12 (10, 13). Contrary to its T cell and macrophage inhibitory actions, IL-10 enhances B cell survival, proliferation, differentiation, and immunoglobulin production (14). Levels of IL-10 production are critical in immune regulation and control the balance between inflammatory and humoral responses. Various studies have confirmed the association of IL-10 with autoimmune and infectious diseases (15).
The human IL-10 gene is located on chromosome 1 and has been mapped to the junction between 1q31 and 1q32 (16). The capacity for IL-10 production is correlated with the genetic composition of the IL-10 locus. Genetic factors account for approximately 75% of the variation in IL-10 secretion capacity in humans, and these genetic differences contribute to disease susceptibility (17). The variation of IL-10 expression is thought to be at the transcriptional level since mutations in the IL-10 gene promoter sequence may alter specific transcriptional activation and cytokine production (18).
This study hypothesized that the IL-10 gene polymorphisms have a measurable influence on KD susceptibility and contribute to or reduce the occurrence of coronary artery lesions. To test this hypothesis, inheritance of the IL-10 promoter polymorphism at position -592 in patients with KD, as well as the association of the polymorphism with clinical complications of CALs, were examined.
We enrolled patients with KD from the Department of Pediatrics at the China Medical University Hospital, Taichung, Taiwan. The study group included 105 patients, all of whom met the criteria proposed by the Japanese Kawasaki Disease Research Committee (19). (See Table 1 for the numbers of subject studied.) All patients were treated with IVIG (2 g/kg infused over 8-12 hr) and oral aspirin (80-100 mg/kg/day). Echocardiographs were obtained by the pediatric cardiologist before or within 2 weeks of IVIG administration. Coronary artery lesions were diagnosed from the echocardiograms using the criteria proposed by the Japanese Kawasaki Disease Research Committee (19): coronary arteries were classified as abnormal if the internal lumen diameter was ≥3 mm in children younger than 5 yr old or ≥4 mm in children older than 5 yr old, if the internal diameter of a segment measured ≥1.5 times that of an adjacent segment, or if the coronary lumen was clearly irregular. We also studied 100 unrelated healthy children (62 males and 38 females, 0.4-7.0 yr old, mean age=3.2 yr) who served as the control group. All blood samples were drawn before IVIG therapy in the KD patient group. Control samples were tested in parallel with patient samples. The ethics committee of the China Medical University Hospital Institutional Review Board approved the study, and written informed consent was obtained from the parents of all subjects.
The genomic DNA was prepared from peripheral blood using a genomic DNA isolation reagent kit (Genomaker, Taipei, Taiwan). The IL-10 A-592C genotype was determined using a polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP) assay. The primers were forward 5'-CCTAGGTCACAGTGACGTGG-3' and reverse 5'-GGTGAGCACTACCTGACTAGC-3'. This generated a PCR product of 412 bp. PCR amplification was done in a 50-µL reaction volume containing 100 ng of genomic DNA, 0.5 µM of each primer, 0.2 mM/L deoxynucleotide triphosphates, 10 mM/L Tris-HCl (pH 8.3), 50 mM/L KCl, 1.5 mM/L MgCl2, and 1.25 units of Taq polymerase (Perkin Elmer, Foster City, CA, U.S.A.). The PCR amplification was performed in a programmable thermal cycler (Gene Amp PCR System 2400; Perkin-Elmer). PCR cycle conditions consisted of an initial denaturation step at 95℃ for 5 min followed by 35 cycles of 1 min at 94℃, 1 min at 60℃, 1 min at 72℃, and a final extension at 72℃ for 6 min. The PCR products were digested with restriction endonuclease RasI (New England Biolabs, Beverly, MA, U.S.A.) and the digested DNA fragments were separated by 3% agarose gel electrophoresis and identified by ethidium bromide staining. In the presence of the IL-10 -592*A allele, RasI cut the 412 bp PCR product into two fragments of 236 and 176 bp, respectively.
The SAS Version 8.2 (SAS Institute Inc., NC, U.S.A.) with chi-square test was used to compare the genotype and the allelic frequency distribution of the IL-10 gene for patients with KD and control subjects. A chi-square test was also used to compare differences between patients with and without coronary artery lesions. A value of P<0.05 indicated a significant difference between the tested populations. Associations between variables in cases and control subjects were determined using the odds ratio and 95% confidence interval.
The characteristics of the 105 KD patients studied are shown in Table 1. The sex distribution of the total KD patients was a male/female ratio of 1.4:1, which is comparable to the previous reports of KD male/female ratios ranging from 1.5 to 1.7:1.31 patients (30%) were identified as having coronary artery lesions. Parents of all KD patients enrolled in the study on a voluntary basis; therefore, the study population is not representative of the true incidence of either KD or coronary artery lesions in Taiwan.
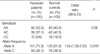
The frequencies of the genotype in the KD and control groups are shown in Table 2. Among the 105 KD patients, 56 (53.3%) had IL-10 genotype AA, 39 (37.1%) had AC, and 10 (9.6%) had CC. Among the 100 control subjects, 40 (40.0%) had genotype AA, 42 (42.0%) had AC, and 18 (18.0%) had CC. There were no significant differences in the genotype distribution of IL-10 A-592C gene polymorphism between the healthy control subjects and KD patients (P=0.08). However, the frequency of the -592*A allele was significantly increased in the patients with KD compared with controls (71.9% vs. 61.0%, P=0.019). The odds ratio for developing Kawasaki disease in individuals with IL-10-592*A allele was 1.64 (95% confidence interval, 1.06-2.52) compared to individuals with the IL-10 -592*C allele. The association of IL-10 A-592C gene polymorphism with coronary artery lesions of KD was examined. Both genotype and allelic frequencies were not statistically different between KD patients with and without coronary artery lesions (Table 3).
Kawasaki disease is an acute, inflammatory, systemic vasculitis of unknown etiology that is often complicated by coronary artery aneurysm. KD is currently thought to be an infectious disease with immunologic manifestations that occur only in genetically susceptible individuals (1). However, the genetic background of patients with KD is mostly unknown. Cytokines with polymorphic gene sequences are potential markers of disease susceptibility because their gene products are involved in KD pathogenesis (2-7). This study provides evidence for a genetic association between KD and the IL-10 gene.
The level of IL-10, one of the anti-inflammatory cytokines, is markedly elevated (as are various proinflammatory cytokines) during the acute phase of KD. IL-10 reduces the inflammatory response of monocytes/macrophages and inhibits cytokine production by Th1 cells. On the other hand, IL-10 is a potent stimulator for human B cells. The significance of increased IL-10 level observed in KD is not fully understood. It may contribute to the upregulation of humoral immune responses (increased B cell activation and hypergammaglobulinemia are observed during the acute phase of KD [20]) and to the downregulation of acute inflammation arising from increased levels of proinflammatory cytokines such as TNF-α(7).
The genetic makeup of the IL-10 promoter can determine the level of IL-10 secretion. The promoter of the IL-10 gene has been shown to be highly polymorphic. Among the identified polymorphisms in the IL-10 promoter, three linked single nucleotide polymorphisms (SNPs) (i.e., -1,082 G/A, -819 T/C, and -592 A/C) have been shown to influence the IL-10 gene expression (21). Several functional studies showed that -592*A, -819*T, and -1,082*A alleles are associated with low levels of IL-10 production (22-24). In addition, earlier reports (23, 24) found complete linkage disequilibrium between allele -819*C and allele -592*C and between allele -819*T and allele -592*A. IL-10 gene polymorphisms, especially the IL-10 A-592C polymorphism, have been implicated in associations with disease outcome. The position -592 is in an area containing putative binding sites for NF-IL-6 and STAT-1 (25). The -592*A allele has been associated with increased incidence of sepsis and mortality in critically ill patients (26) and with renal involvement in SLE patients (27). These results suggest the influence of the IL-10 A-592C polymorphism in disease progression and prognosis.
The prevalence of IL-10 -592*A allele varies from population to population (Table 4), with the greatest rate found in Japanese (67.2%) (28), southern Chinese from Hong Kong (67%) (27), Korean (62%) (29), and British Caucasians (21%) (23, 24). In our study of Chinese healthy subjects from Taiwan, the IL-10 -592*A allele frequency was 61.0%, which is very similar to this frequency in Korean and Hong Kong Chinese but markedly different from Caucasians. The distribution of various genotypes for the IL-10 promoter polymorphic sites differ significantly between ethnic populations suggesting that this could be a useful anthropologic genetic marker. The frequency of the IL-10 -592* A allele was significantly increased in our patients with KD, suggesting that it might represent a candidate genetic marker to predict the development of KD for an individual.
Serum levels of an array of cytokines, including proinflammatory and anti-inflammatory cytokines, are elevated in patients with KD. Complex regulatory interactions exist to suppress ongoing inflammatory response. Regulation is often achieved via parallel secretion of antagonistic cytokines and soluble receptors. However, it is difficult to predict the net contribution of inhibitory cytokines to an inflammatory response by determination of serum levels. Furthermore, such regulation is further complicated by the ratio of cytokine to soluble receptor, such as TNF to sTNFR or IL-10 to sIL-10R, within the local environment. Therefore, we speculated that the relative lack of IL-10 compared with the elevated levels of various proinflammatory cytokines may contribute to the proinflammatory milieu in KD even though increased IL-10 level was observed during the acute phase of KD. Thus, the fact that most Asians carry the IL-10 -592*A allele (which is associated with low IL-10-secreting capacity) may partially explain why Kawasaki disease is most highly expressed in Asian populations, mainly Japanese (30). Function-related experiments and investigation to determine whether IL-10 -592*A allele is similarly associated with KD in subjects of other ethnic origin would be intriguing and worthwhile.
Of interest, a recent study conducted in a Korean population by Jin et al. (31) showed no significant difference in allele or genotype frequency for the IL-10 -592 A/C polymorphism between KD patients and control subjects but the study showed a significantly higher frequency of the IL-10 -592 C allele in KD patients with CALs. Although a relatively small sample size is a limitation of both our study and that by Jin et al., the inconsistent results may be due to environmental factors and differences in the genetic backgrounds of the study populations. Because KD appears to be a polygenic disease, and a wide number of cytokines seem to be implicated in the regulation of vasculitis. It is apparently unlikely that the IL-10 -592 polymorphism is the only gene conferring susceptibility to KD and it remains formally possible that the association may reflect linkage disequilibrium with polymorphisms in currently uncharacterized genes. As a result, although the distribution of IL-10 promoter polymorphisms is very similar in healthy controls from Taiwan and Korea, the other important immunogenetic differences in terms of disease susceptibility and severity between patients from these two populations may also explain the discordant results observed in the IL-10 gene polymorphism distribution.
We were unable to demonstrate a role of this polymorphism in the predisposition of KD patients to the development of CALs. There may be a number of reasons for this. First, the number of patients with CALs was too small to determine a significant association. Second, this polymorphism may simply not be involved in the genetic susceptibility to CALs. Third, specific characteristics of disease pathology within KD, such as CALs formation, may be associated with specific polymorphisms in a number of genes and may represent interacting complex genetic traits. Thus, the role of IL-10 A-592C polymorphism in the development of CALs may be apparent only when it is examined in the context of the interaction of other polymorphisms in or close to IL-10 gene or located in other genes which are related to angiogenic and inflammatory processes.
The present study is somewhat limited by the relatively small study population, the possibility of linkage disequilibrium with an unknown risk single nucleotide polymorphism and the lack of function-related experiments. However, our findings provide preliminary data suggesting that IL-10 -592*A allele is associated with susceptibility to KD in the Taiwanese population. To establish firmly the relationship between IL-10 promoter polymorphism and KD, further large-scale studies are required in individuals of other ethnicities.
Figures and Tables
Table 2
Genotypes and allelic frequencies of IL-10 A-592 C promoter polymorphism in KD patients and normal control subjects
References
1. Wang CL, Wu YT, Liu CA, Kuo HC, Yang KD. Kawasaki disease: infection, immunity and genetics. Pediatr Infect Dis J. 2005. 24:998–1004.
2. Lin CY, Lin CC, Hwang B, Chiang BN. The changes of interleukin-2, tumor necrosis factor, and gamma-interferon production among patients with Kawasaki disease. Eur J Pediatr. 1991. 150:179–182.
3. Lin CY, Lin CC, Hwang B, Chiang B. Serial changes of serum interleukin-6, interleukin-8, and tumor necrosis factor alpha among patients with Kawasaki disease. J Pediatr. 1992. 121:924–926.

4. Matsubara T, Furukawa S, Yabuta K. Serum levels of tumor necrosis factor, interleukin 2 receptor, and interferon-gamma in Kawasaki disease involved coronary-artery lesions. Clin Immunol Immunopathol. 1990. 56:29–36.
5. Hirao J, Hibi S, Andoh T, Ichimura T. High levels of circulating interleukin-4 and interleukin-10 in Kawasaki disease. Int Arch Allergy Immunol. 1997. 112:152–156.
6. Eberhard BA, Andersson U, Laxer RM, Rose V, Silverman ED. Evaluation of the cytokine response in Kawasaki disease. Pediatr Infect Dis J. 1995. 14:199–203.

7. Kim DS, Lee HK, Noh GW, Lee SI, Lee KY. Increased serum interleukin-10 level in Kawasaki disease. Yonsei Med J. 1996. 37:125–130.

8. Noh GW, Lee WG, Lee W, Lee K. Effects of intravenous immunoglobulin on plasma interleukin-10 levels in Kawasaki disease. Immunol Lett. 1998. 62:19–24.

9. Mosmann TR. Properties and functions of interleukin-10. Adv Immunol. 1994. 56:1–2.
10. Fiorentino DF, Bond MW, Mosmann TR. Two types of mouse T helper cell IV. Th2 clones secrete a factor that inhibits cytokine production by Thl clones. J Exp Med. 1989. 170:2081–2095.
11. Taga K, Tosato G. IL-10 inhibits T cell proliferation and IL-2 production. J Immunol. 1992. 148:1143–1148.
12. Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993. 151:1224–1234.
13. De Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin-10 (IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991. 174:1209–1220.
14. Rousset F, Garcia E, Defrance T, Peronne C, Vezzio N, Hsu DH, Kastelein R, Moore KW, Banchereau J. Interleukin-10 is a potent growth and differentiation factor for activated human B lymphocytes. Proc Natl Acad Sci USA. 1992. 89:1890–1893.
15. Moore KW, de Waal Malefyt R, Coffman RL, O'Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001. 19:683–765.
16. Kim JM, Brannan CI, Copeland NG, Jenkins NA, Khan TA, Moore KW. Structure of the mouse IL-10 gene and chromosomal localization of the mouse and human genes. J Immunol. 1992. 148:3618–3623.
17. Westendorp RG, Langermans JA, Huizinga TW, Elouali AH, Verweij CL, Boomsma DI, Vandenbroucke JP. Genetic influence on cytokine production and fatal meningococcal disease. Lancet. 1997. 349:170–173.

18. Eskdale J, Gallagher G, Verweij CL, Keijsers V, Westendorp RG, Huizinga TW. Interleukin 10 secretion in relation to human IL-10 locus haplotypes. Proc Natl Acad Sci USA. 1998. 95:9465–9470.

19. Research Committee on Kawasaki Disease. Report of Subcommittee on Standardization of Diagnostic Criteria and Reporting of Coronary Artery Lesions in Kawasaki Disease. 1984. Tokyo: Ministry of Health and Welfare of Japan.
20. Furukawa F, Ohshio G, Hamashima Y. Possible polyclonal B cell activation in mucocutaneous lymph node syndrome. Eur J Pediatr. 1986. 145:104–108.

21. Eskdale J, Kube D, Tesch H, Gallagher G. Mapping of the human IL10 gene and further characterization of the 5'flanking sequence. Immunogenetics. 1997. 46:120–128.
22. Rosenwasser LJ, Borish L. Genetics of atopy and asthma: the rationale behind promoter-based candidate gene studies (IL-4 and IL-10). Am J Respir Crit Care Med. 1997. 156:S152–S155.

23. Turner DM, Williams DM, Sankaran D, Lazarus M, Sinnott PJ, Hutchinson IV. An investigation of polymorphism in the interleukin-10 gene promoter. Eur J Immunogenet. 1997. 24:1–8.

24. Edwards-Smith CJ, Jonsson JR, Purdie DM, Bansal A, Shorthouse C, Powell EE. Interleukin-10 promoter polymorphism predicts initial response of chronic hepatitis C to interferon alfa. Hepatology. 1999. 30:526–530.

25. Kube D, Platzer C, von Knethen A, Straub H, Bohlen H, Hafner M, Tesch H. Isolation of the human interleukin 10 promoter. Characterization of the promoter activity in Burkitt's lymphoma cell lines. Cytokine. 1995. 7:1–7.

26. Lowe PR, Galley HF, Abdel-Fattah A, Webster NR. Influence of interleukin-10 polymorphisms on interleukin-10 expression and survival in critically ill patients. Crit Care Med. 2003. 31:34–38.

27. Mok CC, Lanchbury JS, Chan DW, Lau CS. Interleukin-10 promoter polymorphisms in Southern Chinese patients with systemic lupus erythematosus. Arthritis Rheum. 1998. 41:1090–1095.

28. Tegoshi H, Hasegawa G, Obayashi H, Nakano K, Kitagawa Y, Fukui M, Matsuo S, Deguchi M, Ohta M, Nishimura M, Nakamura N, Yoshidawa T. Polymorphisms of interferon-γ gene CA-repeat and interleukin-10 promoter region (-592A/C) in Japanese type 1 diabetes. Hum Immunol. 2002. 63:121–128.
29. Chin HJ, Na KY, Kim SJ, Oh KH, Kim YS, Lim CS, Kim S, Chae DW. Interleukin-10 promoter polymorphism is associated with the predisposition to the development of IgA nephropathy and focal segmental glomerulosclerosis in Korea. J Korean Med Sci. 2005. 20:989–993.

30. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004. 110:2747–2771.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download