1. Wallace RJ Jr, Cook JL, Glassroth J, Griffith DE, Olivier KN, Gordin F. American Thoracic Society statement: diagnosis and treatment of disease caused by nontuberculous mycobacteria. Am J Respir Crit Care Med. 1997. 156:S1–S25.
2. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007. 175:367–416.

3. Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005. 20:913–925.

4. Swensen SJ, Hartman TE, Williams DE. Computed tomographic diagnosis of Mycobacterium avium-intracellulare complex in patients with bronchiectasis. Chest. 1994. 105:49–52.

5. Primack SL, Logan PM, Hartman TE, Lee KS, Muller NL. Pulmonary tuberculosis and Mycobacterium avium-intracellulare: a comparison of CT findings. Radiology. 1995. 194:413–417.

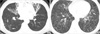
6. Jeong YJ, Lee KS, Koh WJ, Han J, Kim TS, Kwon OJ. Nontuberculous mycobacterial pulmonary infection in immunocompetent patients: comparison of thin-section CT and histopathologic findings. Radiology. 2004. 231:880–886.

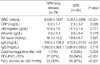
7. Koh WJ, Lee KS, Kwon OJ, Jeong YJ, Kwak SH, Kim TS. Bilateral bronchiectasis and bronchiolitis at thin-section CT: diagnostic implications in nontuberculous mycobacterial pulmonary infection. Radiology. 2005. 235:282–288.

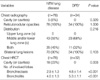
8. Chung MJ, Lee KS, Koh WJ, Lee JH, Kim TS, Kwon OJ, Kim S. Thin-section CT findings of nontuberculous mycobacterial pulmonary diseases: comparison between Mycobacterium avium-intracellulare complex and Mycobacterium abscessus infection. J Korean Med Sci. 2005. 20:777–783.

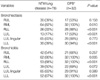
9. Chung MJ, Lee KS, Koh WJ, Kim TS, Kang EY, Kim SM, Kwon OJ, Kim S. Drug-sensitive tuberculosis, multidrug-resistant tuberculosis, and nontuberculous mycobacterial pulmonary disease in non-AIDS adults: comparisons of thin-section CT findings. Eur Radiol. 2006. 16:1934–1941.

10. Azuma A, Kudoh S. Diffuse panbronchiolitis in East Asia. Respirology. 2006. 11:249–261.

11. Akira M, Kitatani F, Lee YS, Kita N, Yamamoto S, Higashihara T, Morimoto S, Ikezoe J, Kozuka T. Diffuse panbronchiolitis: evaluation with high-resolution CT. Radiology. 1988. 168:433–438.

12. Nishimura K, Kitaichi M, Izumi T, Itoh H. Diffuse panbronchiolitis: correlation of high-resolution CT and pathologic findings. Radiology. 1992. 184:779–785.

13. Poletti V, Casoni G, Chilosi M, Zompatori M. Diffuse panbronchiolitis. Eur Respir J. 2006. 28:862–871.
14. Griffith DE, Brown-Elliott BA, Langsjoen B, Zhang Y, Pan X, Girard W, Nelson K, Caccitolo J, Alvarez J, Shepherd S, Wilson R, Graviss EA, Wallace RJ Jr. Clinical and molecular analysis of macrolide resistance in Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2006. 174:928–934.
15. Homma H, Yamanaka A, Tanimoto S, Tamura M, Chijimatsu Y, Kira S, Izumi T. Diffuse panbronchiolitis. A disease of the transitional zone of the lung. Chest. 1983. 83:63–69.
16. Tsang KW, Ooi CG, Ip MS, Lam WK, Ngan H, Chan EY, Hawkins B, Ho CS, Amitani R, Tanaka E, Itoh H. Clinical profiles of Chinese patients with diffuse panbronchiolitis. Thorax. 1998. 53:274–280.

17. Kilian M, Mestecky J, Russell MW. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial immunoglobulin A proteases. Microbiol Rev. 1988. 52:296–303.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download