Abstract
Although the mechanisms are unclear, rush immunotherapy (RIT) may be effective to treat allergic diseases. We investigated the long-term modifications of cellular immunity as a mechanism of RIT. The RIT group, included 15 house dust mite (HDM)-sensitized asthmatic children, received RIT only with Dermatophagoides farinae (Der f) and Dermatophagoides pteronyssinus (Der p), whereas the control group, consisted of 10 HDM-sensitized asthmatic children, did not receive RIT. The asthma symptom scores and the skin reactivities to Der f were measured. The cellular proliferative responses and intracellular interleukin (IL)-5 and interferon (IFN)-γ productions from peripheral blood T cells were also measured before, 8 weeks and 1 yr after RIT. The symptom scores, skin reactivity to Der f and cellular proliferative responses to Der f were decreased significantly after 8 weeks and maintained until 1 yr of RIT. The IFN-γ/IL-5 ratio of the CD3(+) and CD4(+) cells were increased significantly after 8 weeks and maintained until 1 yr of RIT, while there were no changes in the control group. These data indicate that the continuous functional modification from Th2 to Th1 phenotype of the CD4(+) T cells are developed after RIT in the asthmatic children sensitized with HDM.
Allergen specific immunotherapy is the only curative treatment modality available at present and has been used successfully in limited allergic diseases, such as insect venom allergy, allergic rhinoconjunctivitis (1, 2) and asthma (3-5). Controlled long-term trials suggest that immunotherapy has the capacity to modify the natural history of allergic airway disease by reducing the incidence of new sensitization (6-8), reducing allergic symptoms through years after discontinuation and preventing the incidence of asthma (1, 2, 4). But the precise molecular mechanisms underlying this treatment modality remain elusive.
Although previous studies were focused on circulating blocking antibodies (9-11), they could not explain the antigen-specific effects of immunotherapy. Other suggested mechanisms were reducing in the numbers of mast cells and eosinophils including the release of mediators (12-14). Recent studies suggest the modulation of interleukin (IL)-4 and/or interferon (IFN)-γ production from allergen-specific T cells (4, 5, 15-19), especially a decrease in allergen-induced T cell activation and both Th2 (IL-4, IL-5, and IL-13) and Th1 cytokines (IFN-γ), which were induced by the increase of regulatory T cell cytokine production, such as IL-10 and/or transforming growth factor-β (TGF-β) (20-23).
The hypothesis of our study was that immunotherapy may induce the functional modification from Th2 to Th1 phenotype in T cells, and these changes will be induced in early phase of rush immunotherapy (RIT) and continued to late phase. To investigate the modifications of cellular immunity in the mechanism of RIT, we evaluated the production of intracellular IL-5 and IFN-γ from the peripheral blood T cells in the early and late phase of the allergic inflammation in asthmatic children with and without RIT.
Twenty five children with atopic asthma (16 boys and 9 girls) who were sensitive only to Dermatophagoides farinae (Der f) and Dermatophagoides pteronyssinus (Der p) were enrolled. Patients were divided into two groups, such as those who had received RIT (n=15, RIT group) and those who had never received RIT (n=10, control group).
The diagnosis and severity of asthma were determined for each patient by the American Thoracic Society guidelines (24). Asthma was confirmed by a history of dyspnea and wheezing during the previous 12 months, a greater than 12% reversibility of forced expiratory volume in one second (FEV1) spontaneously or after β2-agonist inhalation, and/or a methacholine provocation test result with a PC20 less than 16 mg/mL. All of the patients were belong to mild to moderate asthma. The two groups were comparable for the severity of asthma, the concentration of total immunoglobulin E (IgE) and Der f or Der p-specific IgE, and allergic skin test to Der f or Der p (Table 1). At the time of the study, all patients were symptom free (for at least 4 weeks) and did not have any medication for asthma, except for short acting β2-agonist, for at least 4 weeks before blood sampling. The ethics committee of the Asan Medical Center Institutional Review Board approved the study, and written informed consents were obtained from the parents of all subjects.
The patients received RIT according to the protocol in our pediatric ward. The patients were admitted for 4 days during induction period, and the dosages of each injection were rapidly increased in ten-fold daily starting from 5 Therapeutic unit (TU)/mL. Then they received injections every 2 weeks till 8 weeks until maintenance was achieved (5,000 TU/mL). After then they received injections every 4 weeks. Allergen extracts were obtained from Bencard Allergie (Munchen, Germany).
The respiratory symptom scores of asthma (25) and the skin test reactivities (allergen/histamine ratio) to Der f and Der p were measured. The severity of symptoms was scored on a Likert scale from 0 to 5 (0=none, 1=trivial, 2=mild, 3=moderate, 4=severe, and 5=very severe). Asthma symptom complaints were divided into three periods: awakening (four items), daytime (six items), and nighttime (five items). The total numbers of nocturnal awakenings for asthma per week were translated into the same six-point scale as follows: no awakening=none, 1 awakening=trivial, 2 to 3 awakenings=mild, 4 to 6 awakenings=moderate, 7 to 10 awakenings=severe, and 11 or more awakenings=very severe.
Peripheral blood mononuclear cells (PBMCs) from the patients were centrifugated by Ficoll-Hypaque gradient (Pharmacia, Uppsala, Sweden) and were incubated in 96 well round bottom plate at 106 cells/mL in 200 µL/well. The dosages of Der f allergen (Allergopharma, Reinbek, Germany) were 1, 10, 30, and 50 µg/mL concentration. Proliferative responses were assayed by tritiated thymidine incorporation after 5 day's culture after addition of 1 µCi 16 hr before cell harvesting.
For intracellular T-cell cytokine detection, we employed a flow cytometry, which has been described elsewhere (26). PBMCs (107 cells/mL) were stimulated with phorbol myristate acetate (25 ng/mL; Wako, Osaka, Japan) and ionomycin (1 µg/mL; Sigma Chemical Co., St. Louis, MO, U.S.A.) in the presence of 2 mM/L monensin for 4 hr at 37℃ in a humidified atmosphere of 5% CO2 in air. Cells were fixed in 4% paraformaldehyde and made permeable with saponin. After blocking with 10% AB serum, cells were incubated with a monoclonal mouse anti-cytokine antibody (anti-IL-5 and anti-IFN-γ: Becton-Dickinson, Franklin Lakes, NJ, U.S.A.) at 1 mg/mL in 0.1% saponin. Twenty minutes later, cells were washed twice and incubated with a fluorescein isothiocyanate- or phycoerthrin-labeled polyclonal goat anti-mouse isotype specific antibody and an anti T-cell surface marker antibody (anti-CD3, anti-CD4, and anti-CD8: Becton-Dickinson) for 30 min. Then cells were analyzed by FACSCalibur (Becton-Dickinson) and frequency of cytokine-producing cells in different subpopulation was calculated.
The respiratory symptom scores were decreased significantly 8 weeks after RIT and maintained until 1 yr after RIT (Fig. 1A), but not in control group (data not shown). In addition, the skin test reactivity (allergen/histamine ratio) to Der f was decreased significantly 8 weeks after RIT and maintained until 1 yr after RIT (Fig. 1B), but not in control group (data not shown).
The proliferative response was expressed as stimulation index (SI: cpm with allergen/cpm with medium). The cellular proliferative responses (SI) to each dose of Der f showed dose-dependent responses and the maximum response was found in the stimulation of Der f 30 µg/mL concentration. These responses were decreased significantly 8 weeks after RIT and maintained until 1 yr after RIT (Fig. 2A). However, in the control group the cellular proliferative responses were not suppressed even on 8 weeks and 1 yr follow-up (Fig. 2B).
By flow cytometry, the frequencies of CD4(+) and CD8(+) T cells were analyzed before and after RIT. The CD3(+) cells and CD4(+) cells increased at 8 weeks after RIT, but these cells decreased at 1 yr after RIT compare to the cell population at 8 weeks after RIT (Fig. 3A). In contrast, there were no significant changes in 3 follow-up periods in the control group (Fig. 3B). In addition, the CD8(+) cells were not changed at 8 weeks and 1 yr follow-ups in either groups (data not shown).
Intracellular IFN-γ and IL-5 staining for CD3, CD4, and CD8 were carried out on activated PBMCs. RIT increased IFN-γ/IL-5 ratio from the CD3(+) T cells (data not shown), and from the CD4(+) T cells at 8 weeks after RIT (P<0.01), but not from the CD8(+) T cells (data not shown), compared to those before RIT. IFN-γ/IL-5 ratio was maintained until 1 yr after RIT from the CD4(+) T cells (P<0.01, Fig. 4A), whereas IFN-γ/IL-5 ratio from the peripheral CD3(+), CD4(+) or CD8(+) T cells was not changed in each follow-up period in the control group (Fig. 4B). These results suggest that RIT induced the modification of cellular immune responses in the peripheral T cells, such as Th2 to Th1 shift in the peripheral CD4(+) T cells.
This study shows early increase of IFN-γ/IL-5 ratio from the peripheral blood CD4(+) T cells in children with house dust mite-sensitized asthma even at 8 weeks after RIT. Additionally this change of IFN-γ/IL-5 ratio was maintained till 1 yr after RIT, whereas these findings were not detected in asthmatic children without RIT. These changes were mainly originated from increase of IFN-γ production rather than changes of IL-5 production from the peripheral CD4(+) T cells regardless of RIT. These findings suggest that the immunologic changes after RIT may start early, especially before maintenance therapy, and be originated from the production of the IFN-γ particularly from the CD4(+) T cells, not from the CD8(+) T cells. In addition, these early changes were maintained until 1 yr after RIT. These findings suggest that the Th1 shift on peripheral blood CD4(+) T cells may be one of the main functional modifications of allergen specific immunotherapy and also this effect may be maintained during immunotherapy. Our data is compatible with some of the previous reports (4, 5), whereas some reported that IFN-γ increases while IL-4 diminishes (5, 15, 19) and some others showed that IL-4 was decreased while IFN-γ was not altered (16-18). In addition, others showed a decrease in both cytokines IFN-γ and IL-4 (20, 27). However, these variable results for the immunologic changes after immunotherapy might depend on the protocol of immunotherapy, the type of allergen, the time point of evaluation, the type of stimuli used for cytokine production, and the cell sources used.
The most important change in the allergen-specific immune response during immunotherapy is characterized by a decrease in allergen-induced T cell activation and the production of both Th1 and Th2 cytokines, which outlines peripheral T cell tolerance to allergens. Several studies have shown an increase in regulatory T cells, especially in bee-venom immunotherapy (20, 22, 23). T cell tolerance can be directly initiated by the autocrine action of IL-10 (21, 22), which can inhibit the full maturation of dendritic cell (28). This results in the long lasting induction of tolerance in both Th1 and Th2 cells.
Also, the respiratory symptom were improved significantly in the early phase of RIT only and that was also maintained until 1 yr after RIT. In this study, there was good correlation between the changes of respiratory symptom score and the changes of IFN-γ(+)/CD4(+) cells. In addition, skin reactivity to Der f, which means skin mast cell reactivity, was also significant decrease in the early phase of RIT, and that was persisted until 1 yr after RIT. These findings suggest that not only asthma symptoms but also target tissue responsiveness are improved after RIT, which may be related to the shift of Th1 immune response or decreased histamine release from target cells including basophils and mast cells (14).
IFN-γ-production from peripheral CD4(+) T cells was lower in atopic asthmatics (29). According to these results, immunotherapy may induce the shift of cytokine balance from Th2 to Th1 milieu in the systemic circulation.
RIT suppressed the cellular proliferation in each dose of Der f, and also this suppression was maintained until 1 yr after RIT only in RIT group in contrast to control group. These data were compatible to the previous study (4) which showed decreased proliferation of peripheral blood mononuclear cells to relevant allergen (Der p) in early phase of RIT, but not to irrelevant allergen. Although the inhibition of cellular proliferation underlies the clinical effect of RIT, the mechanism of RIT is not clear. The possibility may be suggested by the induction of allergen-specific suppressor T cells (30), the induction of anergy (31), or the induction of IFN-γ (32).
To confirm the increase of IFN-γ production from the PBMCs after RIT, we also evaluated the serial studies at three different time points during RIT in 7 patients, such as 8 weeks before, immediately before, and 8 weeks after RIT. IFN-γ(+)/CD4(+) T cells were not changed between 8 weeks before and immediately before RIT, but were increased at 8 weeks after RIT (P<0.05, data not shown). These data suggest that IFN-γ(+)/CD4(+) T cells might be increased by the effect of RIT.
The mechanism of increment of IFN-γ producing cells by RIT is not defined. The possible mechanism might be explained by the route of allergen exposure and the amount of administered allergens, which may alter the function of antigen presenting cells (33). The use of repeated high allergen concentrations during RIT may lead the shift to the Th1 cell response. It appears that RIT may correct the decreased IFN-γ production of CD4(+) T cells in the children with atopic asthma.
All our results were originated from the peripheral blood T cells of the asthmatic patients, therefore these did not reveal the direct cause and effect of RIT in the asthmatic airway, so we pointed the systemic effect of RIT. Additionally we suggest that the Th1 shift of immunologic cells may be important in the mechanism of RIT.
Figures and Tables
 | Fig. 1Respiratory symptom scores (A) and skin test reactivity to Der f (B) were decreased significantly at 8 weeks after RIT and maintained until 1 yr after RIT in the RIT group, but not in control group. |
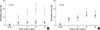 | Fig. 2The cellular proliferative responses (stimulation index, SI) were decreased significantly at 8 weeks after RIT (•) and maintained until 1 yr after RIT (□) compared to those before RIT (○) (A), whereas they were not suppressed in the control group (B). |
 | Fig. 3The CD4(+) T cells were increased at 8 weeks after RIT, but these cells were decreased at 1 yr after RIT compared to the cell population at 8 weeks after RIT (A). There were no significant changes during 3 follow-up periods in the control group (B). |
References
1. Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, Till SJ, Hamid QA, Nouri-Aria KT. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999. 341:468–475.

2. Eng PA, Reinhold M, Gnehm HP. Long-term efficacy of preseasonal grass pollen immunotherapy in children. Allergy. 2002. 57:306–312.

3. Abramson MJ, Puy RM, Weiner JM. Allergen immunotherapy for asthma. Cochrane Database Syst Rev. 2000. 2:CD001186.

4. Lack G, Nelson HS, Amran D, Oshiba A, Jung T, Bradlev KL, Giclas PC, Gelfand EW. Rush immunotherapy results in allergen-specific alterations in lymphocyte function and interferon-γ production in CD4+ T cells. J Allergy Clin Immunol. 1997. 99:530–538.

5. Majori M, Caminati A, Corradi M, Brianti E, Scarpa S, Pesci A. T-cell cytokine pattern at three time points during specific immunotherapy for mite-sensitive asthma. Clin Exp Allergy. 2000. 30:341–347.

6. Des Roches A, Paradis L, Menardo JL, Bouges S, Daures JP, Bousquet J. Immunotherapy with a standardized Dermatophagoides pteronyssinus extract. VI: specific immunotherapy prevents the onset of new sensitizations in children. J Allergy Clin Immunol. 1997. 99:450–453.
7. Pajno GB, Barberio G, De Luca F, Morabito L, Parmiani S. Prevention of new sensitizations in asthmatic children monosensitized to house dust mite by specific immunotherapy: a six-year follow-up study. Clin Exp Allergy. 2001. 31:1392–1397.

8. Moller C, Dreborg S, Ferdousi HA, Halken S, Host A, Jacobsen L, Koivikko A, Koller DY, Niggemann B, Norberg LA, Urbanek R, Valvirta E, Wahn U. Pollen immunotherapy reduces the development of asthma in children with seasonal rhinoconjunctivitis (the PAT-study). J Allergy Clin Immunol. 2002. 109:251–256.
9. Aalbers R, Van der Gaag R, Van Leeuwen J. Serologic aspects of IgG4 antibodies. I. Prolonged immunization results in an IgG4 antibody response. J Immunol. 1983. 130:722–726.
10. Moss RB, Hsu YP, Kwasnicki JM, Sullivan MM, Reid MJ. Isotypic and antigenic restriction of the blocking antibody response to rye grass pollen: correlation of rye group I antigen-specific IgG1 with clinical response. J Allergy Clin Immunol. 1987. 79:387–398.
11. Kim BS, Lee SK, Park HS, Hong SJ. The early changes of humoral immune response after rush immunotherapy with Dermatophagoides farinae and Dermatophagoides pteronyssinus in house dust mite sensitive asthmatic children. J Asthma Allergy Clin Immunol. 2001. 21:543–551.
12. Creticos PS, Adkinson NF Jr, Kagery-Sobotak A, Proud D, Meier HL, Naclerio RM, Lichtenstein LM, Norman PS. Nasal challenge with ragweed pollen in hay fever patients. Effect of immunotherapy. J Clin Invest. 1985. 76:2247–2253.

13. Rak S, Lowhagen O, Venge P. The effect of immunotherapy on bronchial hyperresponsiveness and eosinophil cationic protein in pollen-allergic patients. J Allergy Clin Immunol. 1988. 82:470–480.

14. Shim JY, Kim BS, Cho SH, Min KU, Hong SJ. Allergen-specific conventional immunotherapy decreases IgE-receptor-mediated basophil histamine release. Clin Exp Allergy. 2003. 33:52–57.
15. Jutel M, Pichler WJ, Skrbic D, Urwyler A, Dahinden C, Muller UR. Bee venom immunotherapy results in decrease of IL-4 and IL-5 and increase of IFN-γ secretion in specific allergen-stimulated T cell cultures. J Immunol. 1995. 154:4187–4194.
16. Secrist H, Chelen CJ, Wen Y, Marshall JD, Umetsu DT. Allergen immunotherapy decreases interleukin 4 production in CD4+ T cells from allergic individuals. J Exp Med. 1993. 178:2123–2130.

17. Van Bever HP, Vereecke IF, Bridts CH, De Clerck LS, Stevens WJ. Comparison between the in vitro cytokine production of mononuclear cells of young asthmatics with and without immunotherapy (IT). Clin Exp Allergy. 1998. 28:943–949.

18. Pène J, Desroches A, Paradis L, Lebel B, Farce M, Nicodemus CF, Yssel H, Bousquet J. Immunotherapy with Fel d 1 peptides decreases IL-4 release by peripheral blood T cells of patients allergic to cats. J Allergy Clin Immunol. 1998. 102:571–578.
19. Mamessier E, Birnbaum J, Dupuy P, Vervloet D, Magnan A. Ultra-rush venom immunotherapy induces differential T cell activation and regulatory patterns according to the severity of allergy. Clin Exp Allergy. 2006. 36:704–713.

20. Akdis CA, Akdis M, Blesken T, Wymann D, Alkan SS, Muller U, Blaser K. Epitope-specific T cell tolerance to phospholipase A2 in bee venom immunotherapy and recovery by IL-2 and IL-15 in vitro. J Clin Invest. 1996. 98:1676–1683.

21. Jutel M, Akdis M, Budak F, Aebischer-Casaulta C, Wrzyszcz M, Blaser K, Akdis CA. IL-10 and TGF-beta cooperate in the regulatory T cell response to mucosal allergens in normal immunity and specific immunotherapy. Eur J Immunol. 2003. 33:1205–1214.
22. Akdis CA, Blesken T, Akdis M, Wuthrich B, Blaser K. Role of interleukin 10 in specific immunotherapy. J Clin Invest. 1998. 102:98–106.

23. Pereira-Santos MC, Baptista AP, Melo A, Alves RR, Soares RS, Pedro E, Pereira-Barbosa M, Victorino RM, Sousa AE. Expansion of circulatory Foxp3+CD25brightCD4+ T cells during specific venom immunotherapy. Clin Exp Allergy. 2007. 38:291–297.
24. American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease (COPD) and asthma. Am Rev Respir Dis. 1987. 136:225–244.
25. Wasserfallen JB, God K, Schulman KA, Baraniuk JN. Development and validation of rhinoconjunctivitis and asthma symptom score for use as an outcome measure in clinical trials. J Allergy Clin Immunol. 1997. 100:16–22.
26. Jung T, Schauer U, HeusSeries C, Neumann C, Rieger C. Detection of intracellular cytokines by flow cytometry. J Immunol Methods. 1993. 159:197–207.

27. O'Brien RM, Byron KA, Varigos GA, Thomas WR. House dust mite immunotherapy results in a decrease in Der p 2-specific IFN-γ and IL-4 expression by circulating T lymphocytes. Clin Exp Allergy. 1997. 27:46–51.
28. Steinbrink K, Wolfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997. 159:4772–4780.
29. Kim JH, Kim BS, Lee SY, Seo JH, Shim JY, Hong TJ, Hong SJ. Different IL-5 and IFN-γ production from peripheral blood T cell subsets in atopic and nonatopic asthmatic children. J Asthma. 2004. 41:869–871.
30. Tamir R, Castracane JM, Rocklin RE. Generation of suppressor cells in atopic patients during immunotherapy that modulate IgE synthesis. J Allergy Clin Immunol. 1987. 79:591–598.

31. Lamb JR, Skidmore BJ, Green N, Chiller JM, Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983. 157:1434–1447.

32. Gajewski TF, Fitch FW. Anti-proliferative effect of IFN-γ in immune regulation. I. IFN-γ inhibits the proliferation of Th2 but not Th1 murine helper T lymphocyte clones. J Immunol. 1988. 140:4245–4252.
33. DeKruff RH, Fan Y, Umetsu DT. IL-4 synthesis by in vivo primed keyhole limpet hemocyanin-specific CD4+ T cells. J Immunol. 1992. 149:3468–3476.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download