Abstract
This study was designed to isolate Ca2+-activated K+ current (IKCa) and elucidate its physiological significance in freshly isolated interstitial cells of Cajal (ICCs) of guinea-pig stomach. Single ICC was freshly isolated by enzymatically dissociating from myenteric border of gastric antrum free of circular muscles, and conventional whole-cell voltage clamp technique including immunohistochemical techniques were employed to characterize the cells: In myenteric border of gastric antrum, ICC-MY (ICCs from myenteric border) were detected by immunohistochemical reactivity, and single ICC-MY which has many branches was immunohistochemically c-Kit positive. Under K+-rich and 0.1 mM ethylene glycol-bis (2-aminoethyl ether)-N,N,N',N'-tetraacetic acid pipette solution, ICC produced spontaneous inward current (-256±92.2 pA). When step-depolarizing pulse from -80 to +80 mV was applied at holding potential (Vh) of -80 mV, voltage-dependent outward currents were recorded with superimposed spontaneous transient outward currents (STOCs). Both STOCs and outward currents were reversibly affected by tetraethylammonium chloride (TEA) and iberiotoxin (IbTX); 2 mM TEA and 200 nM IbTX completely abolished STOCs and significantly inhibited outward K+ current over the whole potential range tested for current/voltage (I/V) relationship. In addition, TEA delayed repolarization phase of spontaneous inward current. The present results indicate the presence of IKCa in a single ICC, and it might be involved in regulation of repolarizing phase of spontaneous inward current in guinea-pig stomach.
Spontaneous gastrointestinal (GI) motility is controlled by interstitial cells of Cajal (ICCs), which are known to produce pacemaker potential (1, 2). Spontaneous electric oscillation from ICCs spreads to neighboring GI smooth muscle cells via gap junction and, therefore, ICCs time the phasic contraction such as peristalsis.
ICCs are distributed in various regions of GI tract, and the population of ICCs can be grouped according to their regional distribution: ICC-MY (myenteric border), ICC-IM (intramuscular ICC), ICC-SM (submucosal surface), ICC-DMP (deep muscular plexus), and ICC-SEP (septa between muscle bundle) (3, 4). In general, pacemaker potentials of stomach are generated from ICC-MY as in small intestine and spread to neighboring circular and longitudinal smooth muscles (5, 6), whereas, ICC-IM of stomach and pyloric sphincter is known to form synaptic connection with nerve terminals of enteric motor neurons (7, 8) and plays a crucial role in receiving inputs from motor neurons (9). Therefore, functions of ICCs seem to be diverse and dependent on the organ and/or species (3, 4, 9). Because ICC-MY (or ICC-SM in some species) produces spontaneous inward periodic current, it seems to be the genuine pacemaker cell of GI tract (10-12).
Because not observed in the absence of ICC, slow wave is regarded as a functional expression of ICC. To date, several types of conductance have been isolated as a major contributor for generating spontaneous inward current from ICC; Ca2+-inhibited nonselective cationic and Ca2+-activated Cl- conductance including ether-a-go-go related gene (ERG) K+ channel in cultured murine ICC (13-15). Even [Ca2+]i handling in mitochondria has been suggested as a main mechanism for generating spontaneous activity (16), and periodic intracellular Ca2+ oscillation of ICC has also been reported (17, 18). Further, slow wave of GI tract is affected by removing extracellular Ca2+ (19, 20), and the existence of K+ channels, known to reduce membrane excitability by activating outward K+ current, was already reported in GI tract (21, 22), including Ca2+-activated K+ channel (KCa channel) of canine colonic ICC-SM (11). Therefore, termination of slow wave by activating channel might be one of the possible explaining physiological relevance of this channel in the regulation of GI rhythmicity (23). Indeed, it has been postulated that activation of KCa channel in GI tract reflects repolarization phase of slow wave (24).
However, there are some controversies regarding the role of KCa channel. Tetraethylammonium chloride (TEA), known to block KCa channel, failed to show regulatory effect on murine slow wave (15), and the existence of KCa channel has not yet been verified in either freshly isolated murine network ICC or in cultured one (25). Even in freshly isolated murine ICC network, spontaneous outward K+ current has been reported as a current recorded from fibroblast-like cells (FLCs). In GI tract, physiological function and typical motility pattern show the characteristics of each region; stomach for grinding and small intestine for absorption, thus suggesting that complicated mechanisms might underlie such regional regulation. Considering all the above uncertainties, we felt it necessary to verify the existence of KCa conductance in ICC and characterize its function. Therefore, this study was designed to isolate and elucidate the physiological relevance of KCa current (IKCa) in freshly isolated gastric ICC of guinea-pig.
Guinea-pigs of both gender, weighing 300-350 g, were anesthetized with fluoromethyl 2,2,2-trifluoro-1(trifluoromethyl) ethyl ether (sevoflurane, Maruishi Pharma., Osaka, Japan), and exanguinated after stunning or decapitation. All experiments were performed in accordance with the guidelines for the animal care and use approved by the Chungbuk National University, The Physiological Society of Japan and Shanghai Jiaotong University. The antral portion of stomach was cut, and the mucosal layer was separated from the muscle layers in Ca2+-free physiological salt solution (Ca2+-free PSS). Then, the circular muscle layer of the tissues was carefully removed from the longitudinal layer by sharp dissection with fine scissors under dissecting microscope. Through these processes, we obtained myenteric border upward antral tissue including longitudinal layer. Then, the tissue was cut into small segments (3×3 mm). These segments were incubated in Ca2+-free PSS for an hour at 4℃. Then, they were incubated for 15 min at 36℃ in the digestion medium containing 0.1% collagenase (Wako, Osaka, Japan), 0.05% dithiothreitol, 0.1% trypsin inhibitor and 0.2% bovine serum albumin. After the digestion, the supernatant was discarded, and the softened muscle segments were transferred into Ca2+-free PSS, and single cells were then dispersed by gentle agitation with a wide-bore glass pipette. Isolated gastric ICCs were kept in Ca2+-free PSS at 4℃ until use. All experiments were carried out within 6 hr of harvesting cells. Temperature for conventional whole-cell voltage clamp experiment was controlled at 32℃.
Isolated cells were transferred to a small chamber on the stage of an inverted microscope (IX-70 or IX-71, Olympus, Tokyo, Japan). The chamber was perfused with PSS (2-3 mL/min). Glass pipettes with a resistance of 2-5 MΩ were used to make a giga seal of 5-10 GΩ, by using standard patch clamp techniques (26). Membrane currents were amplified with 200B patch-clamp amplifier (Molecular Devices, Sunnyvale, CA, U.S.A.), and data were digitized with Digidata 1220 or Digidata 1322, stored directly and digitized on-line using pClamp software (version 5.5.1 or 9.2). Data were displayed on a digital oscilloscope and a computer monitor, and data were analyzed using Origin software and pClamp 6.0 (pClamp 9.2).
Ca2+ PSS contained the following (in mM): NaCl 140, KCl 4.5, CaCl2 2, MgCl2 1, glucose 10, and HEPES (N-[2-hydroxyethyl] piperazine-N'-[2-ethanesulphonic acid]) 10. Its pH was adjusted to 7.4 with NaOH. Phosphate-added cold Tyrode's solution contained (mM): NaCl 145, KCl 5, MgCl2 2, CaCl2 2, glucose 10, NaH2PO4 0.42, Na2HPO4 1.81, HEPES 10, pH 7.4. For recordings of whole-cell currents, the pipette solution contained (mM): KCl 140, MgCl2 5, K2ATP 2.7, Na2GTP 0.1, creatine phosphate (disodium salt) 2.5, HEPES 5 and ethylene glycol-bis (2-aminoethyl ether)-N,N,N',N'-tetraacetic acid (EGTA) 0.1. pH was adjusted to 7.2 by Tris. This is referred to as the K+-rich pipette solution in the text. All drugs presently used for electrophysiology were purchased from Sigma.
The stomach was cut along the lower curvature, and the mucosa and circular muscle layers were dissected away. For the detection of c-Kit positive ICC-MY on myenteric border, circular muscular layer from antrum was carefully removed by fine scissors. Preparations were processed to immunohistochemically reveal ICC using an antibody: To visualize cells expressing c-Kit immunoreactivity, tissue and single isolated ICC were incubated for an hour in PSS containing monoclonal antibodies raised against the c-Kit protein (ACK-2, diluted 1:100, eBioscience, San Diego, CA, U.S.A.) (27). Then, tissue was washed 5 times with PSS. And single ICC was centrifused (1,000 r.p.m.) and the pellet was washed 4 times with PSS. Then, tissue and single isolated ICC were incubated for an hour in an anti-rat IgG antibody labeled with a fluorescent marker (goat anti-rat IgG Texas Red, 1:500, Molecular probes, Eugene, OR, U.S.A.). Then, the preparation was pinned out on a Sylgard block (silicone elastomer, Dow Corning Corporation, Midland, MI, U.S.A.) which had window of some 3×3 mm in the center. The Sylgard block was turned over and then placed at the bottom of the chamber, so that the preparation faced a glass coverslip. The distribution of ICC-MY was determined by constructing z-stacks from the myenteric region where networks of c-Kit positive cell were apparent in guinea-pig stomach. The cells were examined with a Leica Type TCS SP2 AOBS confocal microscope (Leica Microsystems, Heidelberg GmbH, Gräfelfing, Germany) under Leica inverted microscope (Leica DMIRE2, Leica, Germany). Optical lense of dry type of 40.0× and NA (0.75) was used (Leica Microsystems). Confocal image was captured with an excitation wave length appropriate for Texas Red (488 nm). Final images were constructed with Leica Confocal Software Ver. 3.0 (Leica Microsystem).
Under confocal microscope, c-Kit immunohistochemical reactiviry was detected in the myenteric border. As shown in Fig. 1A, typical phenotypes such as fusiform cell body and multi processes in the central area of the cell were observed (1, 4, 27). Furthermore, these cells were connected to each other and formed two dimensional networks in myenteric border (n=3). Therefore, it is highly likely that these cells are ICC-MY.
As described above under Method, we also isolate fresh noncultured single ICC-MY from the gastric myenteric border of guinea-pig using simple enzyme treatment. As shown in the right panel of Fig. 1B, single ICC from the myenteric border (the right panel in Fig. 1B) have many processes and it was easily discernable when compared with spindle like and non branched GI smooth muscle cell (the left panel in Fig. 1B). These cells were then incubated with c-Kit antibody and Texas red, and unbound antibodies were washed out at least 4 times by centrifugation (1,000 r.p.m.). As shown in the left pannels of Fig. 1C, isolated single ICC from guinea-pig gastric antral myenteric border showed typical ICC phenotype under phase contrast condition (the left panel in Fig. 1C) (1, 4, 27). In addition, it also expressed c-Kit immunohistochemical reactivity under confocal microscope (the middle and right panels of Fig. 1C). On the other hand, phenotype of smooth muscle was absolutely different from that of ICC and also it did not express c-Kit immunohistochemical reactivity (data not shown). To further confirm the identity of ICC, we also recorded spontaneous inward current from c-Kit positive cell. Under conventional whole-cell voltage clamp and K+-rich pipette solution (Vh=-100 mV), ICC freshly isolated from guinea-pig gastric antrum generated spontaneous inward current with mean amplitude of -256±92.2 pA (n=9; Fig. 1D).
To record and characterize IKCa in single ICC freshly isolated from guinea-pig gastric antrum, conventional whole-cell voltage clamp experiment was performed at 32℃. When step depolarizing pulse from -80 to +80 mV was applied at holding potential of -80 mV, voltage dependent outward current, which superimposed spontaneous transient outward K+ currents (STOCs) on each traces, was recorded (Fig. 2A-C). The peak and steady state outward current recorded at +80 mV were 3,820±666.9 pA and 2,095±417.9 pA, respectively (n=9). STOCs were also observed by application of ramp-hyperpolarizing pulse from +80 to -120 mV at Vh=-60 mV (Fig. 2B). Both outward K+ current and STOCs were significantly inhibited by tetraethylammonium chloride (TEA, 2 mM): 2 mM TEA completely blocked STOCs (Fig. 2C), while outward K+ current was also significantly inhibited in a reversible manner compared to the control (Fig. 2C, D). As shown in Fig. 2D and E, the peak and steady-state outward current recorded at +80 mV were 3,863±1,142 pA and 2,009±604.7 pA, respectively. Furthermore, they were significantly inhibited by 2 mM TEA to 1,109±369.1 pA and 654±190.9 pA, respectively (n=5, respectively; P<0.05). Five mM TEA inhibited these peak and steady-state currents to 593±290.3 pA and 438±194.5 pA, respectively (n=3, data not shown). Iberiotoxin (IbTX), which is known to inhibit IKCa specifically, also inhibited both outward K+ current and STOCs significantly. As shown in Fig. 3A, B, the peak and steady-state outward current recorded at +80 mV were suppressed by IbTX (200 nM) to 24±6.8% and 33±12.9% of the control, respectively (n=3, respectively; P<0.05). Inhibitory effect of TEA and IbTX on IKCa was also observed at whole potential ranges tested in current/voltage (I/V) relationship (Fig. 2E, 3B). In addition, TEA significantly delayed repolarization phase of spontaneous inward current. Fig. 3C shows trace of spontaneous inward current in the presence of 5 mM TEA. When currents at 10 sec after spontaneous inward current reached the peak level were compared in the absence and presence of 5 mM TEA, it was decayed to 9.0±4.4% and 56±13.3% of the control, respectively (n=3; P<0.05). Thus, the present study provides evidence on the existence of IKCa in single isolated ICC and its physiological function for regulation of spontaneous inward current in guinea-pig stomach.
In the present study, we established a method to dissociate and identify a single ICC from guinea-pig gastric antrum. Then we tried to isolate IKCa and study its physiological role in the regulation of slow wave. The results indicated that IKCa exists and that this channel might be involved in the regulation of repolarization of pacemaker current.
ICCs are known to express c-Kit immunohistochemical activity and typical phenotype such as branches (1, 4, 27). Because of these characteristics, ICCs are easily distinguished from that of gastric smooth muscle cells which have spindle-like shape and no branches (Fig. 1B, left panel). Using these criteria, we were able to establish the method for harvesting single ICC-MY from gastric antrum (Fig. 1B, C). However, for further confirmation, it should be verified whether isolated ICCs produce spontaneous inward activity, since pacemaker potentials are known to be generated from ICC-MY in the stomach and small intestine, and then propagate to neighboring smooth muscles, resulting in slow waves and follower potentials (5, 6). In colon, however, ICC-SM as well as ICC-MY are known to be pacemaker cells in some species (11). Therefore we recorded spontaneous inward current using single ICC under whole-cell voltage clamp technique to confirm spontaneous activity from single ICC. For recording of spontaneous inward current, Vh was held at -100 mV to give more strong driving force to cell. Fig. 1D shows spontaneous inward current, and these currents are irregular and sparse in frequency. Similiar characteristics of spontaneous inward current have also been observed in cultured single ICC from murine small intestine and colon (10, 27). Synergism or synchronization of each spontaneous activity from individual cells of network ICC-MY might be plausible explanation for the robust and regular generation of pacemaker potential observed at tissue level. It is also possible that some intracellular molecules and/or cross-talk between intracellular signal transduction pathways might be involved in this process. Further investigation is required to clarify these possibilities.
Since spontaneous inward current from cultured single ICC of small intestine is reported to be irregular (10), most studies on ICCs have been performed using cultured murine network ICCs from 10-12 days-old mice (10). However, single ICC should eventually be isolated fresh from tissue and used, because this process seems to be the best way to elucidate underlying mechanism of human pacemaker activity by stable space clamping (26). To date, only a few electrophysiological studies have been performed by using freshly isolated ICCs from GI tract (11, 25, 28). Even though the results in these studies suggest possibility of studying ICCs at single cell level, it is, nevertheless necessary easier and more stable way to obtain ICCs. Because freshly isolated single ICCs are essential to solve functional GI dysmotility and diseases as well as elucidate pacemaker mechanism. In fact, ICCs play important roles, ranging from generation of pacemaker activity to neuronal mediation for coordinating rhythmic GI motility (5-9). Therefore, significant reduction of ICC density, constructional destruction, or abnormal functions of ICCs have been implicated in many GI diseases. For example, injury and/or decreased number of ICCs with subsequent alteration of motility are suggested to be responsible for clinical human disorders such as constipation, acquired megacolon, inflammation (29, 30) and infection-induced motility disorder (30). These observations together indicate that in vitro culture system for the study of pacemaker mechanism in GI tract might limit our research goal to human level. Therefore, we are extremely fortunate that we successfully built the way for harvesting single non-cultured ICC from guinea-pig stomach.
In both cultured and freshly isolated ICC, several conductances have been reported (13-15). IKCa has already been identified in freshly isolated canine ICC-SM, even though c-Kit immunohistochemical reactivity was not evaluated in that case (11). Goto et al. (25) suggested two types of spontaneous inward and outward current from different types of murine cells in myenteric border tissue, and reported two types of cells from myenteric border: c-Kit-positive CD34-negative cells and c-Kit-negative CD34-positve cells. Furthermore, the generation of spontaneous inward pacemaker current was due to the activation of nonselective cation channel (NSC) from murine intestinal network ICCs (14), and spontaneous outward K+ current was suggested to be responsible for the generation of spontaneous outward current and potential fluctuations in FLCs. However, they did not observe any more major time-dependent and voltage-dependent currents (25). In this report, ICC-MY was shown to have only one major conductance; in other words, ICC-MY and FLCs expressed spontaneous inward and outward current via NSC and K+ channel, respectively. However, voltage-dependent inward Ca2+ currents have already been reported in ICC (27, 28), and IKCa has been isolated in freshly isolated canine ICC (11). In the present study, we also identified ICCs which generate spontaneous inward current, and these ICCs showed IKCa with STOCs superimposed on each traces (Fig. 2A-C). KCa channel is ubiquitous, and has been described in many kinds of GI smooth muscles (21, 23, 31-34). In general, they have large conductance and high density on cell membrane (22, 23). As shown in Fig. 2C-E, outward K+ current recorded from ICC-MY was significantly inhibited by TEA (2 mM). In addition, IbTX (200 nM) inhibited outward K+ current of ICC-MY significantly (Fig. 3A). Since low concentration (2 mM) of TEA is known to block KCa channel in various types of cells including GI smooth muscle cell and IbTX is also known as specific inhibitor of IKCa in smooth muscle cell, we were able to identify IKCa from freshly isolated single ICC-MY which is c-Kit positive (11, 34, 35). Generally, activation of K+ channels is suggested to reduce excitability, and KCa channel has also been suggested to contribute to termination of slow wave in GI tract such as canine colon and gastric antrum (11, 23, 31). This possibility was supported by the observation that duration of slow wave was highly increased by TEA treatment (24). In this study, we identified freshly isolated ICC and IKCa in this study, however, in order to more easily detect single ICC, our protocol has to be improved and upgraded. Since the number of ICC-MY is still less than that of GI smooth muscle, we will also try to apply more specific conditions to cells after the protocol is upgraded in future.
Slow wave of GI tract is not abolished by Ca2+ channel antagonists, however, upstroke velocity and plateau amplitude are known to be reduced by Ca2+ channel antagonists and by reduction of extracellular Ca2+ (19, 20). Membrane depolarization and increase of intracellular Ca2+ observed during slow wave event must necessarily affect KCa channel. Therefore, KCa channel is expected to be involved in the regulation of reploarizing phase in pacemaker event. In the present study, we expected that this TEA-sensitive IKCa could be recorded under low [Ca2+]i buffering condition by intracellular application of 0.1 mM EGTA (Method), and also pursued possible involvement of KCa channel in the regulation of repolarization phase of spontaneous inward current from freshly isolated ICCs. As described in results section, repolarizing phase of spontaneous inward current was significantly delayed in the presence of 5 mM TEA. These results suggest that TEA-sensitive KCa channel in ICC-MY might play a role in shortening current duration, which is probably related to the modulation of repolarization phase. However, this interpretation could be challenged, since there are still controversies about the existence of KCa channel and its physiological role in the regulation of slow wave in mouse (15, 25). Pattern of spontaneous activities of ICCs in network is very different from that of single ICC, including amplitude, frequency and regularity, therefore, direct comparison between single and network ICCs should be difficult. KCa channel might be related to another unknown mechanism in tissue state.
As shown in Fig. 2A-C, we also recorded STOCs in freshly isolated ICC. Generally, localized [Ca2+]i transient (Ca2+ sparks and/or puffs) in smooth muscles is known to be responsible for the activation of Ca2+-activated conductance such as KCa channels and Ca2+-activated Cl- channel (36-38). In most cases, however, KCa channel seems to cause STOCs in smooth muscles (36, 38). In addition, two types of intracellular Ca2+ stores (sarcoplasmic reticulum, SR) have been identified; inositol 1,4,5-triphosphate (InsP3)-induced Ca2+-release (IICR) and Ca2+-induced Ca2+ release (CICR) (39, 40). Furthermore, it is well established that Ca2+ release from intracellular Ca2+ stores causes Ca2+ sparks, and that Ca2+ sparks are tightly linked to STOCs in smooth muscles (36, 38). In GI smooth muscles, periodic [Ca2+]i oscillation in ICC is caused by released [Ca2+]i from IICR, CICR and mitochondria (6, 16, 17). In fact, one of the main conductance-underlying-pacemaker event in ICC seems to be under the control of Ca2+ cycling from SR to mitochondria via IP3 receptor (see the diagram in Kim, et al. [27]) (16). In cerebral artery, Ca2+-sparks elicited STOCs and the resulting hyperpolarization seems to bring about relaxation of smooth muscle (37). Physiologically, Ca2+ sparks were also suggested to play an important role in the stabilizing membrane potential or the generation of contraction (38). As seen in Fig. 2C, STOC in ICC was totally abolished by application of 2 mM TEA and 200 nM IbTX (data not shown), indicating the existence of STOCs in ICC freshly isolated from GI tract. Unfortunately, however, we could not evaluate the physiological significance of STOCs recorded from ICC in this study; it might also play a role in the repolarization and/or stabilizing of membrane potential.
To our best knowledge, this is the first report to demonstrate successful isolation of non-cultured c-Kit positive cells from the myenteric border of guinea-pig antrum. Furthermore, we recorded TEA- and IbTX-sensitive IKCa with STOCs which might be involved in the regulation of repolarization phase of spontaneous inward current. This study is expected to be useful in elucidating underlying mechanisms of pacemaker response in ICCs of adult animals including human.
Figures and Tables
Fig. 1
Identification of single ICC by phenotype and c-Kit immunohistochemical reactivity from dispersions of guinea-pig gastric antrum. Single ICC was freshly isolated from myenteric border of gastric antrum of guinea-pig by a simple enzyme treatment. (A) ICC-MY was detected by c-Kit immunohistochemical reactivity from myenteric border of guinea-pig stomach. Scale bar in (A) as 40 µM. Arrows and arrow-heads indicate cell body and branches of ICC, respectively. (B) shows phenotype of freshly isolated single ICC (right panel), and its morphology was compared to that from gastric smooth muscle (left panel). Scale bars in (B) were 40 µM. In panel (C), ICC from myenteric border of guinea-pig expressed c-Kit immunohistochemical reactivity. (C) shows phenotype (left panels) and c-Kit immunohistochemical reactivity (middle and right panels) of ICC. (D) ICC produced spontaneous inward currents.
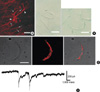
Fig. 2
Ca2+-activated K+ current (IKCa) in single ICC of guinea-pig gastric antrum. (A) At holding potential of -80 mV, application of 20 mV depolarizing step pulses from -100 mV to +80 mV produced outward current which superimposed spontaneous transient outward currents (STOCs) on each traces. (B) STOCs were also recorded in ICC by application of ramp-hyperpolarizing pulse (from +80 to -120 mV). (C) Shows inhibitory effect of TEA (2 mM) on outward current and STOCs. TEA totally blocked STOCs and significantly inhibited outward current. (D) Bar graph (left panel) shows summarized data on the effect of TEA on initial and steady state BK currents. (E) Current-voltage (I/V) relationships of BK current before and after treatment of ICC with TEA.
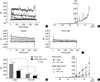
Fig. 3
IKCa and its role for regulation of spontaneous inward current of guinea-pig gastric antral single ICC. At holding potential of -80 mV, outward current was recorded by step depolarizing pulse from -80 to +80 mV. The peak and steady state outward current recorded at +80 mV were significantly inhibited by iberiotoxin (IbTX, 200 nM) in a reversible manner. (A) Bar graph shows summarized data on the effect of IbTX on initial and steady state BK currents. (B) At holding potential of -80 mV, application of 20 mV depolarizing step pulses from -100 mV to +80 mV produced outward current. Current-voltage (I/V) relationships of BK current before and after treatment of ICC with TEA. (C) Effect of TEA on repolaization phase of spontaneous inward current.
References
1. Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/Kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995. 373:347–349.

2. Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the protooncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. J Physiol. 1994. 480:91–97.

3. Chen H, Redelman D, Ro S, Ward SM, Ördög T, Sanders KM. Selective labeling and isolation of functional classes of interstitial cells of Cajal of human and murine small intestine. Am J Physiol Cell Physiol. 2007. 292:C497–C507.

4. Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2005. 68:307–343.

5. Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999. 514:515–531.

6. Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. J Physiol. 2000. 525:105–111.

7. Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proc Natl Aca Sci USA. 1996. 93:12008–12013.

8. Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998. 115:314–329.

9. Ward SM, McLaren GJ, Sanders KM. Interstitial cells of Cajal in the deep muscular plexus mediate enteric motor neurotransmission in the mouse small intestine. J Physiol. 2006. 573:147–159.

10. Koh SD, Sanders KM, Ward SM. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J Physiol. 1998. 513:203–213.

11. Langton P, Ward SM, Carl A, Norell MA, Sanders KM. Spontaneous electrical activity of interstitial cells of Cajal isolated from canine proximal colon. Proc Natl Acad Sci USA. 1989. 86:7280–7284.

12. Thomsen L, Robinson TL, Lee JC, Farraway LA, Hughes MJ, Andrews DW, Huizinga JD. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat Med. 1998. 4:848–851.

13. Huizinga JD, Zhu Y, Ye J, Molleman A. High-conductance chloride channels generate pacemaker currents in interstitial cells of Cajal. Gastroenterology. 2002. 123:1627–1636.

14. Koh SD, Jun JY, Kim TW, Sanders KM. A Ca(2+)-inhibited non-selective cation conductance contributes to pacemaker currents in mouse interstitial cell of Cajal. J Physiol. 2002. 540:803–814.
15. Zhu Y, Golden CM, Ye J, Wang XY, Akbarali HI, Huizinga JD. ERG K+ currents regulate pacemaker activity in ICC. Am J Physiol Gastrointest Liver Physiol. 2003. 285:G1249–G1258.
16. Ward SM, Ördög T, Koh SD, Baker SA, Jun JY, Amberg G, Monaghan K, Sanders KM. Pacemaking in interstitial cells of Cajal depends upon calcium handling by endoplasmic reticulum and mitochondria. J Physiol. 2000. 525:355–361.

17. Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SR, Imaizumi Y, Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci. 2004. 117:2813–2825.
18. Nakayama S, Ohya S, Liu HN, Watanabe T, Fruzono S, Wang J, Nishizawa Y, Aoyama M, Murase N, Matsubara T, Ito Y, Imaizumi Y, Kajioka S. Sulphonylurea receptors differently modulate ICC pacemaker Ca2+ activity and smooth muscle contractility. J Cell Sci. 2005. 118:4163–4173.
19. Fujii K, Inoue R, Yamanaka K, Yoshitomi T. Effects of calcium antagonists on smooth muscle membranes of the canine stomach. Gen Pharmacol. 1985. 16:217–221.

20. Sanders KM, Publicover NG. Chultz S, Rauner BB, Wood JD, editors. Electrophysiology of gastric musculature. Handbook of Physiology: the Gastrointestinal System. 1989. Bethesda, MD: American Physiology Society;187–216.
21. Benham CD, Bolton TB, Lang RJ, Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986. 371:45–67.

22. Carl A, Sanders KM. Ca2+-activated K+ channels of canine colonic myocytes. Am J Physiol. 1989. 257:C470–C480.
23. Carl A, Frey BW, Ward SM, Sanders KM, Kenyon JL. Inhibition of slow-wave repolarization and Ca2+-activated K+ channels by quaternary ammonium ions. Am J Physiol. 1993. 264:C625–C631.
24. Szurszewski JH. A study of the canine gastric action potential in the presence of tetraethylammonium chloride. J Physiol. 1978. 277:91–102.

25. Goto K, Matsuoka S, Noma A. Two types of spontaneous depolarizations in the interstitial cells freshly prepared from the murine small intestine. J Physiol. 2004. 559:411–422.

26. Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp technique for high resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981. 391:85–100.
27. Kim YC, Koh SD, Sanders KM. Voltage-dependent inward currents of interstitial cells of Cajal from murine colon and small intestine. J Physiol. 2002. 541:797–810.
28. Lee HK, Sanders KM. Comparision of ionic currents from interstitial cells and smooth muscle cells of canine colon. J Physiol. 1993. 460:135–152.
29. Lu G, Qian X, Berezin I, Telford GL, Huizinga JD, Sarna SK. Inflammation modulates in vitro colonic myoelectric and contractile activity and interstitial cells of Cajal. Am J Physiol. 1997. 273:G1233–G1245.

30. Wang XY, Vannucchi MG, Nieuwmeyer F, Ye J, Faussone-Pellegrini MS, Huizinga JD. Changes in interstitial cells of Cajal at the deep muscular plexus are associated with loss of distention-induced burst-type muscle activity in mice infected by Trichinella spiralis. Am J Pathol. 2005. 167:437–453.

31. Carl A, McHale NG, Publicover NG, Sanders KM. Participation of Ca2+-activated K+ channels in electrical activity of canine gastric smooth muscle. J Physiol. 1990. 429:205–221.
32. Ohya Y, Kitamura K, Kuriyama H. Cellular calcium regulates outward currents in rabbit intestinal smooth muscle cell. Am J Physiol. 1987. 152:C401–C410.

33. Singer JJ, Walsh JV Jr. Characterization of calcium activated potassium channels in single smooth muscle cells using patch-clamp technique. Pflügers Arch. 1987. 408:98–111.
34. Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by β-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol. 2005. 288:C1255–C1268.
35. Benham CD, Bolton TB, Lang RJ, Takewaki T. The mechanism of action of Ba2+ and TEA on single Ca2+-activated K+-channels in arterial and intestinal smooth muscle cell membranes. Pflügers Arch. 1985. 403:120–127.
36. Bolton TB, Imaizumi Y. Spontaneous transient outward currents in smooth muscle cells. Cell Calcium. 1996. 20:141–152.

37. Nelson MT, Cheng H, Rubart M, Santana LF, Bonev AD, Knot HJ, Lederer WJ. Relaxation of arterial smooth muscle by calcium sparks. Science. 1995. 270:633–637.

38. ZhuGe R, Sims SM, Tuft RA, Fogarty KE, Walsh JV Jr. Ca2+ sparks activated K+ and Cl- channels, resulting in spontaneous transient currents in guinea-pig tracheal myocytes. J Physiol. 1998. 513:711–718.
39. Ganitkevich VY, Isenberg G. Contribution of Ca2+ induced Ca2+ release to the [Ca2+]i transients in myocytes from guinea pig urinary bladder. J Physiol. 1992. 458:119–137.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download