Abstract
Polyfibromatosis syndrome is a rare disease entity that is characterized by various clinical features such as palmar, plantar, and penile fibromatoses, keloid formations of the skin, and erosive arthropathy. Its precise pathophysiology or etiology remains unclear. In addition to distinctive diverse skin manifestations, patients with polyfibromatosis have been previously reported to show erosive arthropathy with significant limitation of movement at affected joints. However, the presence of erosive polyarthropathy in polyfibromatosis has not emphasized in previous cases. Here, we report a case of polyfibromatosis syndrome combined with painless massive structural destruction of hand and foot joints, and review the characteristics of erosive arthropathy in previous cases.
Polyfibromatosis syndrome is a rare disease with unknown etiology, and is characterized by cutaneous keloid formation, fibromatoses of diverse sites including palmar, plantar, penile, and knuckle areas, and erosive changes of the hand and foot joints (1, 2). In 1945, Tourain et al. (3) first described hereditary polyfibromatosis as a rare syndrome that mainly presented with various connective tissue changes. Pierard et al. (1) identified osteolysis of joints in a patient with phenytoin-related polyfibromatosis. Histopathological findings of erosive arthropathy were first reported in a patient with an aggressive form of polyfibromatosis syndrome (4). A follow-up presentation in the same patient was described after 10 yr without any suggestions of effective treatments or definite etiology (5). The precise pathogenesis and clinical or radiographic characteristics of this disease entity remain unclear, although some investigators have attempted to identify the pathogenesis in respect to genetic backgrounds or mechanical properties (6, 7).
Clinical and radiological characteristics for erosive arthropathy in polyfibromatosis have rarely been described, although comments about the presence of arthropathy in some cases have been presented in the literature (1, 4, 5, 8). Here, we described the radiological and clinical features for erosive arthropathy in our case and reviewed erosive arthropathy for cases with polyfibromatosis syndrome in previous reports.
A 44-yr-old male visited our rheumatology clinic because of painless progressive deformities of his hands and feet, in addition to multiple dysmorphic skin lesions with keloid formation on the whole body, especially the chest wall and extremities. Over the next eight years, painless deformities in his hands and feet gradually progressed, leading to the severe palmar and plantar contractures that were seen at his first visit to our clinic. He denied any signs of either articular or periarticular inflammation, such as heat, swelling, or tenderness, during the course of this condition. His family history and drug history were unremarkable.
Keloid formations were visible on his left shoulder, anterior chest wall, both arms, and right leg due to previous injuries including BCG vaccination, thoracostomy site, and blunt traumas. There were ulcerated keloid skin lesions on both of his feet, to which he denied any antecedent trauma. His hands and feet revealed severe flexion deformities without signs of active inflammation in any of his joints. Nodules in his palm and sole also were also identified.
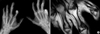
The complete blood count, liver and renal function tests, muscle enzymes, and urinalysis of the patient were normal. Antinuclear antibody, antineutrophilic cytoplasmic antibody, and rheumatoid factor tests were negative, and acute phase reactants such as erythrocyte sediment rate (ESR) and c-reactive protein (CRP) were also within normal range. Pulmonary function tests and nailfold capillary microscopy did not show abnormal findings. Plain radiography of both hands revealed multiple erosive lesions with sclerotic margins or a punch-out appearance in his metacarpophalangeal (MCP) and proximal interphalangeal (PIP) joints and resorptive changes (acrolysis) in distal phalanges (Fig. 1A). A coronal T1-weighed magnetic resonance image (MRI) demonstrated erosive changes at the MCP and PIP joints of the right hand after radio-contrast administration (Fig. 1B). Biopsies of the cutaneous lesions on the trunk showed dermal fibrous proliferation consistent with keloid formations (Fig. 2).
We diagnosed the patient with polyfibromatosis syndrome due to evidence of visible typical skin lesions with compatible pathologic findings on multiple sites and articular or periarticular manifestations such as joint deformities accompanied with skin contractures and nodular formations. Even though we have tried various treatments over the past years, including D-penicillamine, intralesional and medium-dose oral steroids, colchicine, and intravenous cyclophosphamide, none of these have been effective on either his cutaneous or articular manifestations.
Polyfibromatosis syndrome is known for complex fibrosing conditions including knuckle pads, palpable fibrotic nodules of penile area (Peyronie's disease), plantar fibromatosis (Ledderhose's disease), plamar fibromatoses (Dupuytren's contractures), and keloid formations (3). The pathogenesis of polyfibromatois syndrome itself has not yet been determined, although research on the pathophysiology of some components in polyfibromatosis syndrome such as Dupuytren's contractures and Peyronie's disease are relatively well-described (9, 10).
Here, we focused on the massive erosive arthropathy in polyfibromatosis syndrome (Table 1). Polyfibromatosis syndrome accompanied with erosive polyarthropathy is a rare dermatoarthropathy (1, 4, 5, 8) after the first historical description for polyfibromatosis syndrome by Tourain et al. (3) in 1945. It seems that the presence of arthropathy in a patient with phenytoin-related polyfibromatosis was first described by Pierard et al. (1) in 1979, although Black et al. (4) used the term 'erosive arthropathy' in their description. Pierard et al. first described a 20-yr-old Caucasian male with polyfibromatosis and erosive arthropathy associated with phenytoin-dependent fibrosis, who presented with diverse skeletal abnormalities including camptodactyly, facial bone hypoplasia, scoliosis, and osteolytic changes of the wrists, hands, and feet joints (1). Then, histopathologic findings on bone biopsy from a finger with erosive arthropathy in a 48-yr-old Western Indian man with aggressive polyfibromatosis was described by Black et al. (4). The biopsy results showed an increased number of osteoclasts associated with erosion of trabecular bone. Recently, a novel case of polyfibromatosis syndrome combined with interstitial granulomatous dermatitis and arthritis was also reported in the literature (8). Radiologic findings in a new form of an Australian case were also similar with the previous cases of polyfibromatosis syndrome. This case included the first description of radiographic features in erosive arthropathy of polyfibromatosis syndrome by advanced radiologic imaging modalities such as MRI.
The pathogenesis underlying the combined articular and cutaneous manifestations seen in aggressive polyfibromatosis syndrome is unknown. There may be common triggering factors causing not only an abnormal balance between the proliferation and apoptosis of dermal fibroblasts leading to superficial fibromatosis and keloid formation (11, 12), but also that of synovial fibroblasts stimulating the differentiation and activation of osteoclasts that lead to joint erosion (13).
Possible differential diagnosis for punch-out bony erosion and osteolysis in the radiographic findings of this patient without any symptomatic signs or symptom of joint inflammation may include Gorhan-stout syndrome, chronic gout arthritis, nodulosis, arthropathy, osteolysis (NAO) syndrome, or neuropathic arthropathy. Gorham-stout syndrome is a rare disorder that is characterized by massive bone matrix loss with swelling or pain in an affected joint, and is mainly involved in a single joint (14). The distribution of involved joints and MRI findings with enhancement of synovia, marrow, and soft tissue after contrast administration in Gorhan-stout syndrome differed from those in polyfibromatosis syndrome without enhanced lesions. In addition, we could exclude chronic gouty arthritis in diagnosis with respect to the normal range of serum uric acid and absence of typical clinical manifestations of gout. NAO syndrome has an unknown origin that mainly presents with nodulosis and arthropathy with deformed joints (15). This syndrome could be differentiated from our case because it was an autosomal recessive trait, showed early onset of disease, and was responsive to medication. Considering the prevalent sites of involved joints or the presence of underlying diseases such as syphilis, syringomyelia, or diabetes, neuropathic arthropathy could be excluded.
With respect to the treatment of polyfibromatosis syndrome, although various treatments were tried, including steroids, potassium para-aminobenzoic acid, D-penicillamine, methotrexate, minoxidil, and isotretinoin, none of these therapies showed efficacy during a follow-up period of up to 10 yr (4, 5). Our case was also treated with D-penicillamine, intralesional and medium-dose oral steroids, colchicine, and intravenous cyclophosphamide, but these trials resulted in no response.
We proposed a possible association between polyfibromatosis syndrome and erosive arthropathy from the results of our literature review. Our case offers information on another disorder in which rheumatological and dermatological manifestations occurred together. Thus, physicians should be aware of the considerable overlap between the two specialties and increase collaborations to elucidate an etiologic link and, hopefully, an effective treatment.
Figures and Tables
Fig. 1
(A) Hand radiography showing multiple erosions with sclerotic margin at the PIP and MCP joints with flexion deformities of the hand due to Dupuytren's contractures. Resorptive changes (acrolysis) at distal phalanges of both hands were also noted. (B) Coronal T1-weighted spin-echo image (TR/TE 600/29) of the right hand showed multiple bony erosions at the MCP and PIP joints and fatty replacement in soft tissue.
References
1. Pierard GE, Lapiere CM. Phenytoin dependent fibrosis in polyfibromatosis syndrome. Br J Dermatol. 1979. 100:335–341.

2. Gonzalez-Martínez R, Marín-Bertolín S, Amorrortu-Velayos J. Association of knuckle pads and palmo-plantar and penile contracture as clinical manifestations of polyfibromatosis. Eur J Plast Surg. 1998. 21:101–102.
3. Touraine A, Ruel H. La polyfibromatose héréditaire. Ann dermat et syph. 1945. 5:1–5.
5. Lee YC, Chan HH, Black MM. Aggressive polyfibromatosis: a 10 year follow-up. Australas J Dermatol. 1996. 37:205–207.

6. Montgomery E, Lee JH, Abraham SC, Wu TT. Superficial fibromatoses are genetically distinct from deep fibromatoses. Mod Pathol. 2001. 14:695–701.

7. Pierard GE, Lapiere CM. Physiopathological variations in the mechanical properties of skin. Arch Dermatol Res. 1977. 260:231–239.

8. Chen DL, Chong AH, Green J, Orchard D, Williams R, Clemens L. A novel case of polyfibromatosis and interstitial granulomatous dermatitis with arthritis. J Am Acad Dermatol. 2006. 55:Suppl 2. S32–S37.

9. Qian A, Meals RA, Rajfer J, Gonzalez-Cadavid NF. Comparison of gene expression profiles between Peyronie's disease and Dupuytren's contracture. Urology. 2004. 64:399–404.

10. Jemec B, Grobbelaar AO, Wilson GD, Smith PJ, Sanders R, McGrouther DA. Is Dupuytren's disease caused by an imbalance between proliferation and cell death? J Hand Surg [Br]. 1999. 24:511–514.

11. Meek RM, McLellan S, Reilly J, Crossan JF. The effect of steroids on Dupuytren's disease: role of programmed cell death. J Hand Surg [Br]. 2002. 27:270–273.
12. Luo S, Benathan M, Raffoul W, Panizzon RG, Egloff DV. Abnormal balance between proliferation and apoptotic cell death in fibroblasts derived from keloid lesions. Plast Reconstr Surg. 2001. 107:87–96.

13. Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, Goldring SR. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000. 43:250–258.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download