Abstract
Gender differences in asthma have been observed with a preponderance of boys affected before puberty and girls during and after puberty. The known influences of the menstrual cycle on asthma support a role for female sex hormones on the changing expression of asthma during adolescence. The purpose of this study was to investigate obesity, the menstrual cycle and lung function in adolescent girls. One hundred and three female high school girls (mean age: 15.9±0.8 yr) were enrolled. The investigation was performed using a questionnaire that included history of asthma, the menstrual cycle, other combined allergic disease and obesity. The skin prick and pulmonary function test during menstruation period and non-menstruation period. Analyses of these factors were compared. The forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) was significantly lower in the obese group compared to the non-obese group (99.8±13.8 vs. 107.1±10.2, p=0.03). The FEV1 was significantly lower in the girls during menstruation period than in the girls who were not on menstruation (77.5±10.2 vs. 80.4±8.6, p=0.03). Our results showed that changes of pulmonary function were related to menstrual cycle and obesity in Korean adolescent girls.
It is well known that the asthma incidence is greater in boys during childhood and in girls during adolescence. There is no explanation for the change in gender related atopic disease to date. However, hormonal differences and their changes during adolescence have been considered to be contributing factors. It is well known that some female asthmatic patients experience aggravation of asthma symptoms during the premenstrual or menstrual phase of their cycle. This has been referred to as perimenstrual asthma (PMA). PMA has been documented in 30% to 40% of asthmatic women. This form of asthma can be severe and even life threatening with fatal cases were often reported. Therefore, prevention and treatment of PMA is of great interest. However, the underlying mechanisms associated with asthma exacerbation during the perimenstrual period and the related hormones have not been described (1-4).
Several reports have noted associations of body mass index (BMI), asthma and lung function. Reports on the association of early menarche and increasing body weight with asthma suggest that hormonal changes influence the incidence and severity of asthma. However, study findings are inconsistent and the underlying pathophysiology remains unknown (5, 6). Few studies have addressed the possible factors that influence asthma and lung function in adolescent girls. Therefore, in this study, we have explored the factors that influence lung function in adolescent girls focusing on the hormonal factors related to the menstrual cycle and obesity.
High school girls from Seoul, Korea were recruited for this study. We visited one high school three times between May and June of 2006. Enrolled 103 subjects who could cooperate with lung function test on both the second and third visit among 135 girls that completed a questionnaire on the first visit. The mean age was 15.9±0.8 yr and the range was 15-18 yr.
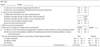
The subjects answered a detailed questionnaire regarding symptoms. Information from physicians included the diagnosis of asthma, body weight and height for BMI, menstrual cycle, age at menarche, change of body weight and height after menarche for calculating change of BMI after menarche, aggravation of asthma symptoms during the perimenstrual period, present and past history of allergic disease, and smoking habits (Table 1).
Asthma: The subjects who had a positive answer to either, "Have you ever had been diagnosed with asthma?" or "Have you ever had attacks of breathlessness at rest with wheezing?" were classified as asthmatics and the others with a negative answer were considered non-asthmatics.
BMI: BMI was calculated as body weight (kg)/height2 (m2). We classified subjects into ≥25 (obesity), 23-24.9 (overweight), 18.5-22.9 (normal), <18.5 (underweight) according to the criteria from the Korean Society for the Study of Obesity.
Menstrual cycle and age at menarche: We collected information on the average number of days of the menstrual cycle and menstrual periods, regularity and dysmenorrhea. We defined early menarche as menarche at 11 yr or earlier.
Increased BMI after menarche: For examine whether an increased BMI after menarche influenced asthma or lung function we compared the current BMI with that at menarche.
PMA: PMA was defined as a self-reported worsening of asthma symptoms during the perimenstrual phase (6). The subjects with a positive answer to the question of "Have you ever had experienced aggravation of asthma symptoms before or during menstruation period?" or "Have you ever had experienced attacks of breathlessness at rest with wheezing before or during menstruation period?" were classified as the PMA group.
Present and past history of other combined allergic disease: We collected information on the present and past history of atopic dermatitis, allergic rhinitis or urticaria.
Smoking: Depending on the smoking history, subjects were divided into 3 categories: current smokers, ex-smokers, non-smokers.
The skin prick test included house dust mites (Dermatophagoides farinae; Df, Dermatophagoides pteronyssinus; Dp), Aspergillus fumigatus, and dog hair; all subjects were tested on the second visit. We used histamine (1 mg/mL) as a positive control and normal saline as a negative control. The skin prick test was performed by placing a drop of 4 extracts and 2 control solutions on the volar surface of the forearm after the area was prepared with 75% ethanol and dried. The drops were placed 2 cm or more apart to avoid a false positive reading. A lancet was passed through the drop, inserted into the epidermal surface, and then gently lifted upward. After 15-30 min a wheal diameter of ≥3 mm or larger than the histamine control was considered a positive result (7).
Lung function test was performed twice for each subject during the second and third visit in order to analyze the changes in lung function according to the menstrual cycle. We used a portable micro-spirometer (Super Spiro, Micro Medical Ltd., Kent, U.K.) and provided instructions prior to the test. At each visit, whether subjects were menstruating was recorded.
Statistical analyses were performed with the SPSS software, version 12.0 (SPSS Inc., Chicago, IL, U.S.A.). Univariate analyses were conducted using the Student's t-test to compare the mean of lung function test results between each of the groups. A paired t-test was used for comparison of the mean of lung function test results during the perimenstrual phase and non-perimenstrual phase of the menstrual cycle. The non-parametric Wilcoxon signed rank test was performed to analyze differences between the mean of lung function test results during the perimenstrual phase and non-perimenstrual phase of the menstrual cycle in the PMA group. p values <0.05 were considered significant.
The average age of the subjects was 15.9±0.8 (range 15-18) and 49 (47.6%) had the diagnosis of asthma among the 103 subjects. The average of BMI was 20.5±2.9. The number of underweight students (22.3%) is greater than obese students (10.7%). There were 62.1% of subjects who were smokers including current smokers (38/103, 36.9%) and ex-smokers (26/103, 25.3%). The average age of menarche was 11.9±1.1 and 32 (31.1%) girls had early menarche (<12 yr old). The subjects answered questions on weight and height at the time of menarche to evaluate an increase in BMI after menarche, however 34 among the 103 could not remember. Sixty-two (78.5%) among 79 girls who did remember showed that the present BMI was increased compared to at menarche. Among the 49 asthmatics, 11 (22.5%) had symptoms of perimenstrual asthma (Table 2).
Among the 11 subjects with BMI of 25 or more, there were three (27.3%) with the diagnosis of asthma this was a smaller percentage than the 46 (50%) among the 92 with BMI less than 25. Four students (36.4%) had menarche at an age less than 12 among 11 in the obese group and 28 (30.4%) among 92 in the non-obese group. Six students (54.5%) were sensitized to inhalant allergens among 11 in the obese group. This was a two fold higher compared to the 21 (22.8%) out of 92 in the non-obese group.
There were 3 (6.1%) obese students with BMI of 25 or more among the 49 subjects with the diagnosis of asthma; this was less than the 8 (14.8%) among the 54 non-asthmatics. The numbers of the girls with early menarche (<12 yr old) was similar in asthmatics and non-asthmatics (30.6% and 31.5%). The students who had an increase of their BMI after menarche were 59.2% in asthmatics and 61.1% in non-asthmatics.
The smokers was 65.3% in asthmatics and 59.3% in non-asthmatics. The number of the students who were sensitized to inhalant allergens was similar in asthmatics and non-asthmatics (26.5% in asthmatics and 25.9% in non-asthmatics). The students who had atopic dermatitis, allergic rhinitis or urticaria in their history were more common in the asthma group (51% in asthmatics and 38.9% in non-asthmatics) and 53.1% students with atopic disease currently had asthma and 27.8% in non-asthmatics.
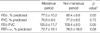
The average percent of predicted forced expiratory volume in one second (FEV1), forced vital capacity (FVC), FEV1/FVC, and forced expiratory flow from 25 to 75% of the vital capacity (FEF25-75%) in the group with BMI of 25 or more compared to the group with BMI less than 25 were the following: 75.9±12.1 vs. 77.3±9.4 (p=0.65), 79.5±13.6 vs. 75.6±11.0 (p=0.30), 99.8±13.8 vs. 107.1±10.2 (p=0.03), 64.6±18.1 vs. 73.7±17.6 (p=0.10). The mean percent of predicted FEV1/FVC was significantly different in the comparison between these two groups (Table 3).
The average percent of predicted FEV1, FEV1/FVC, FVC, and FEF25-75% in smokers compared to non-smokers were 76.1±9.3 vs. 78.9±10.1 (p=0.16), 74.4±11.2 vs. 78.8±11.0 (p=0.06), 107.5±10.1 vs. 104.3±11.6 (p=0.15), 73.0±17.4 vs. 72.3±18.7 (p=0.84).
The average percent of predicted FEV1, FEV1/FVC, FVC, and FEF25-75% in the group with other allergic disorders (atopic dermatitis, allergic rhinitis or urticaria) compared to the group without allergic disease were 77.8±10.1 vs. 76.8±9.4 (p=0.63), 78.5±12.0 vs. 74.5±10.6 (p=0.08), 103.3±11.5 vs. 108.2±9.9 (p=0.03), 69.6±18.4 vs. 74.8±17.3 (p=0.15). The average percent of predicted FEV1/FVC was significantly different in comparisons between these two groups.
The average percent of predicted FEV1, FEV1/FVC, FVC, and FEF25-75% in the group sensitized to inhalant allergens (positive skin prick test to house dust mites (Df and Dp), A. fumigatus, or dog hair) compared to those without sensitization was 76.0±9.4 vs. 79.9±10.8 (p=0.08), 74.6±10.1 vs. 79.9±13.7 (p=0.04), 104.6±12.8 vs. 106.7±10.5 (p=0.40), 71.9±18.8 vs. 74.2±17.1 (p=0.59). The average percent of predicted FEV1/FVC was significantly different in the comparisons between these two groups.
Lung function tests were performed twice in each subject at two-week intervals. The students who could be tested during both the menstrual phase and non-menstrual phase were 51 among the total group of 103. The average percent of predicted FEV1, FEV1/FVC, FVC, and FEF25-75% during menstruation compared to the non-menstruating phase were 77.5±10.2 vs. 80.4±8.6 (p=0.03), 76.8±9.5 vs. 77.2±9.5 (p=0.72), 105.8±11.7 vs. 108.4±8.0 (p=0.05), 72.7±18.1 vs. 76.5±16.0 (p=0.09). The average percent of predicted FEV1 was significantly different in the comparisons between these two groups (Table 4). Among 11 students who reported symptoms of perimenstrual asthma, 6 could be tested both during menstruation and during the non-menstrual phase. Comparison of their lung function during the menstrual phase and non-menstrual phase showed that FEV1/FVC and the average percent of predicted FEF25-75% were significantly lower during the menstrual phase (p=0.03) (Table 5).
The prevalence of obesity in this study group was 10.7% similar to the prevalence of 10.2% previously reported in Korean girls in 2005 by Kim (8). Kim et al. reported that in comparison to the data reported in 1998, body weight and BMI in all ages of girls under 20 yr increased with the exception of girls over 19 who showed a trend towards reduced BMI; suggesting a recent social trend toward undue value of body shape among young people (8, 9). In this study, underweight girls accounted for 22.3% of the subjects, more than twice the number of obese girls.
There are several reports that found an increase in BMI to be associated with an increase in the prevalence of asthma especially in women (11-15). Chang et al. (16) found obesity (BMI ≥25) to be a risk factor for asthma in Korean women. In this study the girls who were diagnosed with asthma, or reported symptoms of asthma, were less prevalent in the obese group (BMI ≥25) than in the non-obese group and the obese girls were less common among the asthmatics compared to the non-asthmatics. However, the results of the lung function test showed that the FEV1/FVC in the obese group was significantly lower than in the non-obese group. These findings are consistent with the report by Hancox (11) who found a significant inverse association between BMI and FEV1/FVC in females. Several mechanisms can explain why obesity is a risk factor for asthma. First, obesity may affect asthma directly by causing decreased tidal excursion leading to smooth muscle latching, and indirectly by enhancement of gastro-esophageal reflux. Second, "leptin", a proteohormone produced by adipocytes, which shows a positive correlation with BMI, has receptors in human lung tissue and provides a link between inflammation and T-cell function in asthma (12, 13). One reason for a stronger relationship between BMI and asthma prevalence in women compared to men and children before puberty may be that women have more body fat mass compared to men with the same BMI and this is related to estrogen (14, 15). Hancox (11) and Weiss (15) reported that BMI and atopy were correlated. In our study, we found sensitization to inhalant allergens to be twice as common in the obese group compared to the non-obese group.
In addition, we found that smokers accounted for 62.1% of asthmatics more than the non-asthmatics. Several studies have reported that smoking is a risk factor for asthma, atopy and bronchial hyper-responsiveness and that it contributes to an increase in the incidence of asthma in women. Furthermore, smoking causes a more severe decline in lung function in asthmatics (17, 18). Our findings showed that the FEV1 and FVC were lower in smokers than in non-smokers but the differences were not statistically significant (p=0.16, p=0.06).
The average age of menarche was found to be 12.6±0.9 in 15 yr olds in Ansan city, in 2001 (19). In this study, it was lower, 11.9±1.1. Salam (20) reported that women with menarche before age 12 had a 2.08-fold higher risk of asthma after puberty, and suggested that this is related to the sex hormones; estrogen and progesterone. One report found that an increased BMI in young female adults contributed to a new diagnosis of asthma (18). In our study, an early menarche or a raised BMI after menarche had no association with asthma. However, our study findings are based on a self-reported questionnaire. The results may be different if we could determine more accurately the onset of asthma and evaluate subjects over a longer time. Allergic disease such as atopic dermatitis, allergic rhinitis and urticaria were more common in the asthmatics than in the non-asthmatics and the FEV1/FVC was significantly lower in the group with allergic disease than in the group without allergic disease. We found that the ratio of sensitization to inhalant allergen was 26.2% and this was a little higher in asthmatics. Sensitization to inhalant allergens has a strong correlation with asthma, allergic rhinitis and bronchial hyper-responsiveness it contributes to an increase in asthma severity and a decrease in lung function (21-24). We found that the FVC was significantly lower in the group with sensitization to inhalant allergens.
Studies suggest that 30 to 40% of asthmatic women report significant exacerbation of asthma symptoms, increase in inhaled short acting beta 2-agonist use or decrease in morning peak flow rate during the perimenstrual phase (25, 26). Furthermore, the asthma attacks related to menstruation are more severe and may be fatal. There are concerns that PMA may be increasing (27, 28). However, the underlying mechanism of PMA remains unclear. There are several hypotheses on the cause of PMA. First, the role of sex hormones on bronchial smooth muscle and β2-adrenergic receptor function. Progesterone potentiates the relaxation effect of β2-agonists on bronchial smooth muscle in vitro. The expected increase in sex-steroid hormones, in non-asthmatic female patients during the luteal phase of the menstrual cycle, is followed by an increase in lymphocyte β2-adrenergic receptor density. However, this regulatory function appears to be lost in women with asthma, who instead appear to show a down regulation of β2-adrenergic receptor density when exposed to progesterone. Second, female sex hormones have effects on several cells and cytokines involved in inflammation. Peripheral blood total white blood cell counts are increased, and a marked deviation of the TH1/TH2 balance toward TH2 occurs during the luteal phase of menstruation. Third, fluid retention is another possible pathophysiologic factor associated with PMA. Increased perimenstrual hydration gives rise to swelling of subcutaneous tissue of the extremities and other parts of the body, such as the bronchial mucosa. This might lead to edema and airway narrowing. In addition, it has been suggested that prostaglandins related to estrogen levels have a role in PMA. Moreover, it has been suggested that progesterone enhances hyperventilation and psychological factors during the premenstrual period and influence respiratory symptoms (6, 28, 29).
PMA is defined as an increase in asthma symptoms or a decrease in lung function during the perimenstrual phase. There is one report that there were significantly more near fatal asthma episodes on the first day of menstruation (28). However, another study reported that emergency room visits for acute asthma were most common during the preovulatory phase (from 5th day to 11th day) (29). When we planed this study, we intended to determine the exact day of the menstrual cycle at the time of lung function test, but a large number of subjects had irregular menstrual cycles and several students could not remember the first day of their last menstrual phase. Therefore, we could compare lung function test only divided by the menstrual phase and the non-menstrual phase. We found that the FEV1 during the menstrual phase was lower than the non-menstrual phase and this difference was statistically significant. Furthermore, FEV1/FVC during the menstrual phase compared to the non-menstrual phase was found to show a very large difference. Moreover, in the group that reported symptoms of perimenstrual asthma, FEV1/FVC and FEF25-75% were significantly lower during the menstrual phase. These findings suggest a correlation between the menstrual cycle and lung function in Korean adolescent girls. Therefore, it may be important to take into consideration hormonal changes related to the menstrual cycle when treating asthmatic adolescent girls.
There is limited information on the association of asthma, lung function and puberty. Our findings provide initial basic data in Korean high school girls. Our results showed that airflow limitation was present in the obese group, in girls with allergic disorders and during the menstrual phase as well as in girls who were sensitized to inhalant allergens. Further investigation into the relationship of sex hormones, leptin, lung function and asthma is needed in adolescent girls.
Figures and Tables
Table 3
Pulmonary function tests in adolescent girls association with obesity in 103 adolescent girls

References
1. Siroux V, Curt F, Oryszczyn MP, Maccario J, Kauffmann F. Role of gender and hormone-related events on IgE, atopy, and eosinophils in the epidemiological study on the genetics and environment of asthma, bronchial hyperresponsiveness and atopy. J Allergy Clin Immunol. 2004. 114:491–498.

2. Becklake MR, Kauffmann F. Gender differences in airway behaviour over the human life span. Thorax. 1999. 54:1119–1138.

3. Osman M. Therapeutic implications of sex differences in asthma and atopy. Arch Dis Child. 2003. 88:587–590.

4. Skobeloff EM, Spivey WH, St Clair SS, Schoffstall JM. The influence of age and sex on asthma admissions. JAMA. 1992. 268:3437–3440.

5. Varraso R, Siroux V, Maccario J, Pin I, Kauffmann F. Asthma severity is associated with body mass index and early menarche in women. Am J Respir Crit Care Med. 2005. 171:334–339.

6. Vrieze A, Postma DS, Kerstjens HA. Perimenstrual asthma: a syndrome without known cause or cure. J Allergy Clin Immunol. 2003. 112:271–282.

7. Brown WG, Halonen MJ, Kaltenborn WT, Barbee RA. The relationship of respiratory allergy, skin test reactivity, and serum IgE in a community population sample. J Allergy Clin Immunol. 1979. 63:328–335.

9. Lee K, Sohn H, Lee S, Lee J. Weight and BMI over 6 years in Korean children: relationships to body image and weight loss efforts. Obes Res. 2004. 12:1959–1966.

10. Romieu I, Avenel V, Leynaert B, Kauffmann F, Clavel-Chapelon F. Body mass index, change in body silhouette, and risk of asthma in the E3N cohort study. Am J Epidemiol. 2003. 158:165–174.

11. Hancox RJ, Milne BJ, Poulton R, Taylor DR, Greene JM, McLachlan CR, Cowan JO, Flannery EM, Herbison GP, Sears MR. Sex differences in the relation between body mass index and asthma and atopy in a birth cohort. Am J Respir Crit Care Med. 2005. 171:440–445.

12. Tantisira KG, Weiss ST. Complex interactions in complex traits: obesity and asthma. Thorax. 2001. 56:Suppl 2. ii64–ii73.
13. Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004. 114:254–259.

14. Shaheen SO, Sterne JA, Montgomery SM, Azima H. Birth weight, body mass index and asthma in young adults. Thorax. 1999. 54:396–402.

15. Weiss ST, Shore S. Obesity and asthma: directions for research. Am J Respir Crit Care Med. 2004. 169:963–968.
16. Chang KJ, Koo HS, Lee HS, Jo YA. Association between asthma and obesity in national health and nutrition survey. J Asthma Allergy Clin Immunol. 2005. 25:262–268.
17. Thomson NC, Chaudhuri R, Livingston E. Asthma and cigarette smoking. Eur Respir J. 2004. 24:822–833.
18. Beckett WS, Jacobs DR Jr, Yu X, Iribarren C, Williams OD. Asthma is associated with weight gain in females but not males, independent of physical activity. Am J Respir Crit Care Med. 2001. 164:2045–2050.

19. Jung YK, Soh JH, Pee DH, Shin YK, Lee KH, Eun BL, Park SH. A study of menarche and dysfunctinal uterine bleeding in adolescent school girls in Ansan city. J Korean Pediatr Soc. 2002. 45:16–23.
20. Salam MT, Wenten M, Gilliland FD. Endogenous and exogenous sex steroid hormones and asthma and wheeze in young women. J Allergy Clin Immunol. 2006. 117:1001–1007.

21. Sritipsukho P. Aeroallergen sensitivity among Thai children with allergic respiratory diseases: a hospital-based study. Asian Pac J Allergy Immunol. 2004. 22:91–95.
22. Peat JK, Tovey E, Toelle BG, Haby MM, Gray EJ, Mahmic A, Woolcock AJ. House dust allergens. A major risk factor for childhood asthma in Australia. Am J Respir Crit Care Med. 1996. 153:141–146.
23. Leung TF, Lam CW, Chan IH, Li AM, Ha G, Tang NL, Fok TF. Inhalant allergens as risk factors for the development and severity of mild-to-moderate asthma in Hong Kong Chinese children. J Asthma. 2002. 39:323–330.

24. Van der Heide S, Dubois AE, Kauffman HF, de Monchy JG. Allergy to mites: relation to lung function and airway hyperresponsiveness. Allergy. 1998. 53:48 Suppl. 104–107.
25. Shames RS, Heilbron DC, Janson SL, Kishiyama JL, Au DS, Adelman DC. Clinical differences among women with and without self-reported perimenstrual asthma. Ann Allergy Asthma Immunol. 1998. 81:65–72.

27. Eliasson O, Scherzer HH, DeGraff AC Jr. Morbidity in asthma in relation to the menstrual cycle. J Allergy Clin Immunol. 1986. 77:87–94.

28. Martinez-Moragon E, Plaza V, Serrano J, Picado C, Galdiz JB, Lopez-Vina A, Sanchis J. Near-fatal asthma related to menstruation. J Allergy Clin Immunol. 2004. 113:242–244.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download