Abstract
The prevalence of reflux esophagitis is increasing in Korea. To estimate the prevalence and clinical characteristics of reflux esophagitis in healthy subjects, we retrospectively examined the medical records of healthy subjects undergoing a routine check-up from October 2004 to September 2005. A total of 6,082 (3,590 men, mean age 44±10 yr) subjects were enrolled in this study. The prevalence of reflux esophagitis in healthy subjects was 10.5%. According to the univariate analysis, male sex (odds ratio [OR] 3.49, 95% confidence interval [CI] 2.84-4.30), smoking history (OR 1.91, 95% CI 1.60-2.28), body mass index (BMI) >30 kg/m2 (OR 2.13, 95% CI 1.37-3.33), total cholesterol >250 mg/dL (OR 1.50, 95% CI 1.05-2.14), low-density lipoprotein (LDL) cholesterol ≥160 mg/dL (OR 1.52, 95% CI 1.08-2.14), triglyceride ≥150 mg/dL (OR 1.92, 95% CI 1.61-2.30), high blood pressure (BP) (OR 1.46, 95% CI 1.20-1.76), and fasting glucose ≥110 mg/dL (OR 1.45, 95% CI 1.13-1.86) were significantly associated with reflux esophagitis (all p<0.05). However, age, alcohol drinking and Helicobacter pylori infection were not associated with reflux esophagitis. In conclusiosn, significant relationships of reflux esophagitis with obesity, low high-density lipoprotein (HDL) cholesterol, high triglyceride, high BP, and elevated fasting glucose suggested that reflux esophagitis might represent the disease spectrum of the metabolic syndrome.
Gastroesophageal reflux disease (GERD) is a major clinical problem in Western countries. Several recent endoscope-based studies have suggested that the overall prevalence of reflux esophagitis (RE) in Western countries was around 10-20% (1). In contrast, GERD has traditionally been considered to be less common in Asia (2). However, more recent studies suggest that the prevalence of GERD in Asia is increasing. In Japan, the overall prevalence of RE among the adult population is roughly 16% (3). In Taiwan, the prevalence of RE in patients evaluated for upper gastrointestinal tract symptoms is 15% (4). These findings are similar to those reported in the West.
RE has been considered fairly rare among ethnic Koreans. However, recent studies have shown that the incidence is increasing in the Korean population. The prevalence of RE in subjects undergoing a routine check-up was reported to 2.36% in 1993 (2), and 3.4% in 1997 (5). In 1999, the prevalence of RE was found to be 5.3% in subjects with gastrointestinal symptoms (6). The incidence is expected to increase not only due to developments in endoscopic examination and increasing awareness of the condition, but also because of changes in preference to a more Westernized diet, as well as changes in lifestyle. However, despite awareness of the increasing prevalence of RE, few studies have been performed to investigate the risk factors of RE in Korea.
This study was performed to estimate the prevalence of RE in healthy Korean subjects and to elucidate the differences in clinical characteristics between subjects with RE and those without.
We retrospectively examined the medical records of healthy subjects who were examined by endoscopy in a routine check-up program of a single university hospital from October 2004 to September 2005. A total of 6,082 subjects were enrolled in the study. Because this was a retrospective study, reflux symptoms such as heartburn, dyspepsia, epigastric pain, and belching were not investigated. Evaluation about smoking and alcohol drinking was performed. The presence of Helicobacter pylori (H. pylori) infection was diagnosed by rapid urease test (Asan Helicobacter test, Asan pharm.co., LTD., Seoul, Korea) or histologic findings from the biopsy specimens. The esophagogastroduodenoscopy (EGD) was a component of a complete medical examination that includes routine studies of blood, urine, stool, and an ultrasound of the abdomen. The body mass index (BMI) was calculated using the following formula: BMI=weight (kg)/height2 (m2). Data for blood pressure (BP), total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglyceride, and fasting glucose were also collected. High BP was defined as ≥130/85 mmHg or documented use of antihypertensive therapy according to National Cholesterol Education Program (NCEP) criteria (7).
Metabolic syndrome was defined based on the World Health Organization (WHO) criteria and NCEP criteria. Under the WHO criteria, the diagnosis of metabolic syndrome can be made in subjects with type 2 DM, impaired glucose tolerance, or insulin resistance, and also requires at least two of the following four components: 1) hypertension, either treated with medication or ≥160/90 mmHg untreated; 2) dyslipidemia with elevated plasma triglyceride (≥150 mg/dL) and/or low HDL (<35 mg/dL in men, <39 mg/dL in women); 3) obesity with BMI ≥30 kg/m2 or central adiposity (waist-hip ratio >0.90 in men or >0.85 in women); and 4) microalbuminuria (urinary average excretion rate ≥20 µg/min or albumin-creatinine ratio ≥20 mg/g). The NCEP criteria for metabolic syndrome require at least three of the following: waist circumference >40 inch in men or >35 inch in women, plasma triglyceride ≥150 mg/dL, HDL cholesterol <40 mg/dL in men or <50 mg/dL in women, blood pressure ≥130/85 mmHg, and fasting plasma glucose ≥110 mg/dL (7).
The severities of RE were defined by the Los Angeles classification (1). The criteria for the diagnosis of esophagitis were: grade A, one or more mucosal breaks confined to the mucosal folds, each no longer than 5 mm; grade B, at least one mucosal break more than 5 mm long confined to the mucosal folds; grade C, at least one mucosal break continuing between the tops of two or more mucosal folds but not circumferential; grade D, circumferential mucosal break. Minimal change esophagitis was excluded because of low interobserver agreement (8).
The differences of mean value in age, BMI, total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, and fasting glucose were evaluated using Student's t test. Categorical variables such as sex, smoking, alcohol drinking and H. pylori infection were evaluated using Pearson chi-square test. The risk of reflux esophagitis was calculated by logistic regression analysis with regards to several variables, including age, sex, smoking, alcohol drinking and H. pylori infection, BMI, high BP, total cholesterol, LDL cholesterol, HDL cholesterol, triglyceride, and fasting glucose. A p value below 0.05 was considered to be statistically significant. The software package used for analysis was SPSS version 13.0 (SPSS Inc., Chicago, IL, U.S.A.).
Among a total of 6,082 subjects (3,590 male and 2,492 female, mean age 44±10 yr, 17-83 yr) (Fig. 1), 639 subjects were found to have RE, and the overall prevalence was 10.5%. The prevalence of RE in male was 14.6% (523 of 3,590), the prevalence of RE in female was only 4.7% (116 of 2,376) (p<0.01). When the age group was stratified by 17-29, 30-39, 40-49, 50-59, 60-69, and 70-83 yr, the prevalence of RE ranged from 10.4% to 15.8% in each age group among male. In contrast, the prevalence of RE ranged 3.7% 9.4% in each age group among female. The prevalence of RE was similar in each age group between both sexes (Fig. 2).
Most of the subjects showed mild grade RE with 519 (81%) of the subjects presenting with grade A, followed by 112 (17.5%) with grade B and 8 (1.3%) with grade C. No subjects presented with grade D (Table 1).
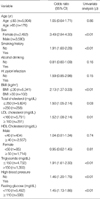
The difference in mean age between subjects with and without RE was not statistically significant (43.9±9.6 yr vs. 43.2±9.7 yr, p=0.5). Male sex was predominant in subjects with RE (81.8%) than those without (56.4%) (p<0.01). Smoking history was significantly higher in subjects with RE (34.6%) than those without (21.6%) (p<0.01). However, alcohol drinking (91.7% vs. 89.9%), and H. pylori infection (2.7% vs. 4.1%) were not significantly different between the two groups. And BMI was also found to be significantly higher in subjects with RE than in those without (24.7±2.8 kg/m2 vs. 23.6±2.9 kg/m2, p<0.01), as were total cholesterol (191.2±33.6 mg/dL vs. 188.3±32.6 mg/dL, p=0.04), LDL cholesterol (112.2±28.1 mg/dL vs. 109.7±27.5 mg/dL, p=0.03), triglyceride (134.6±80.1 mg/dL vs. 113.5±83.2 mg/dL, p<0.01), high BP (25.6% vs. 19.1%, p<0.01), and fasting glucose (98.1±16.7 mg/dL vs. 96.3±18.8 mg/dL, p=0.02). HDL cholesterol (50.7±12.1 mg/dL vs. 53.5±12.7 mg/dL, p<0.01) was significantly lower in subjects with RE than in those without (Table 2).
According to univariate analysis, male sex (odds ratio [OR] 3.49, 95% confidence interval [CI] 2.84-4.30), smoking history (OR 1.91, 95% CI 1.60-2.28), BMI >30 kg/m2 (OR 2.13, 95% CI 1.37-3.33), total cholesterol >250 mg/dL (OR 1.50, 95% CI 1.05-2.14), LDL cholesterol ≥160 mg/dL (OR 1.52, 95% CI 1.08-2.14), triglyceride ≥150 mg/dL (OR 1.92, 95% CI 1.61-2.30), high BP (OR 1.46, 95% CI 1.20-1.76), and fasting glucose ≥110 mg/dL (OR 1.45, 95% CI 1.13-1.86) were significant risk factors of RE (all p<0.05). However, old age (age >65 yr, OR 1.05, 95% CI 0.64-0.71), alcohol drinking (OR 0.81, 95% CI 0.60-1.09) and H. pylori infection (OR 1.59, 95% CI 0.85-2.98) were not shown to be significant risk factors of RE. Low HDL cholesterol (male <40 mg/dL, female <50 mg/dL) was not a significant risk factor in males (OR 1.04, 95% CI 0.81-1.34) and females (OR 0.95, 95% CI 0.62-1.45) (Table 3).
GERD is one of the most prevalent digestive diseases experienced in Western countries. In the United States, the prevalence is about 1,900,000 cases/yr and the annual cost of diagnosis and treatment is 93,000,000,000 dollars (9). The prevalence of GERD-related complications, including RE, Barrett's esophagus, and esophageal adenocarcinoma has been steadily increasing in the United States and Western Europe. As the reasons for the increase in GERD and its complications, changes in diet, smoking, and alcohol intake as well as the declining prevalence of H. pylori infection have been proposed (10). In our results, smoking was an important risk factor, however, alcohol drinking and H. pylori infection had no relationships with GERD. Studies have also hypothesized that the increasing trend of obesity in Western countries has paralleled the increase in esophageal adenocarcinoma and may be an important factor in this change (11).
In Korea, it is generally accepted that the nationwide prevalence of RE is lower than that in Western countries. However, recent studies have shown that the numbers of patients with RE have shown an increasing trend (2, 5, 6). In this study, endoscopic examination indicated that the overall prevalence of RE was 10.5% close to that of Western countries (10-20%) (1). The possible causes for the increasing prevalence in RE observed in the Korean population are of great interest. Patient selection, increased referrals for endoscopy, and marked improvement in diagnostic techniques may partially explain this trend. Furthermore, obesity shows an increasing trend as a cause of RE in Korea. A recently reported study indicated that the prevalence of overweight (25≤BMI≤30 kg/m2) and obesity (BMI >30 kg/m2) was 23.4% and 1.7% in males and 24.9% and 3.2% in females in 1998 (11). However, in 2001, the prevalence of overweight or obesity in Korean adults was 30.6% (32.4% in males and 29.4% in females) (12). In our study, the prevalence of overweight and obesity were 36.9%, 2.1% in males and 21.3%, 2.1% in females, respectively, and BMI was found to be significantly higher in subjects with RE than that in those without (24.7±2.8 kg/m2 vs. 23.6±2.9 kg/m2, p<0.01). BMI >30 kg/m2 was found to be a significant risk factor of RE by univariate analysis (OR 2.13, 95% CI 1.37-3.33).
Obesity may affect esophageal reflux through mechanical factors and humoral factors. Such mechanical factors include increased intra-abdominal pressures, impaired gastric emptying, decreased lower esophageal sphincter pressure, and increased frequency of transient lower esophageal sphincter relaxation, thus leading to increased esophageal acid exposure (10). The humoral mechanisms are not well known. It has been hypothesized that estrogen also influences reflux in obese patients. Two studies conducted by the same group of investigators from Sweden observed that the association between obesity and GERD might be mediated by estrogen (13, 14). The first study reported a statistically significant association between obesity and esophagitis in women, which was potentiated by the use of estrogen in postmenopausal women (13). The second study found that obese women were at increased risk of experiencing symptoms of GERD compared with obese men, and the risk was highest in premenopausal women and postmenopausal women receiving estrogen therapy (14).
Few reports have assessed the association between dyslipidemia and GERD. Total cholesterol was found to be higher in subjects with RE with borderline significance than in the general population, but HDL cholesterol levels did not differ (15). However, in this study, we found that dyslipidemia can be a possible causative factor of RE. Using univariate logistic regression analysis, it was found that total cholesterol >250 mg/dL, LDL cholesterol ≥160 mg/dL, and triglyceride ≥150 mg/dL were associated with the occurrence of RE. HDL cholesterol was significantly lower in subjects with RE than in those without the condition, but low HDL cholesterol (male <40 mg/dL, female <50 mg/dL) was not found to be a significant risk factor. This could potentially be explained by relatively low proportion of the subject's population with low HDL cholesterol.
It is known that the prevalence of RE in adults increases with age (16, 17). In this study, the difference in mean age between subjects with and without esophagitis was not statistically significant. This is may be due to the enrollment of a relatively young subject population (age ≤ 5: n=5,904 vs. age >65: n=178). When we divided the age by subgroup, there were no significant differences within the both sexes. There is a male preponderance in RE, which ranges from 2:1 to 3:1 in comparison to females (18). The preponderance of males with RE in the present study was more evident (M:F=4.5:1). According to univariate analysis, male sex was significant risk factor of RE (OR 3.49, 95% CI 2.84-4.30).
Hypertension has been reported as frequently present in patient with RE (15). The present study showed that high BP was risk factor of RE as well (OR 1.46, 95% 1.20-1.76), but we did not investigate drug therapy in the subjects who underwent endoscopic examination. Calcium antagonists are used to treat hypertension, but these drugs also decrease the lower esophageal sphincter pressure and inhibit muscle contraction in the esophagus itself (19). In Korea, calcium antagonists are widely used to treat hypertension, so it is possible that antihypertensive therapy had an influence on our findings.
An association between diabetes mellitus (DM) and GERD has been reported previously (20, 21). There are several possible mechanisms underlying DM-related GERD. In asymptomatic diabetic patients, there is a higher prevalence of abnormal gastroesophageal reflux than among the general population (22). The incidence of symptomatic GERD tended to be higher in diabetic patients with neuropathy than in those without neuropathy (20). However, the relationship between glucose intolerance and GERD has not yet been reported. In the present study, fasting glucose was significantly higher in subjects with RE than in those without (98.1±16.7 mg/dL vs. 96.3±18.8 mg/dL, p=0.02), and fasting glucose ≥110 mg/dL was found to be a significant risk factor of RE (OR 1.45, 95% CI 1.13-1.86). Hyperglycemia may influence esophageal motor and sensory function (23). In healthy volunteers, hyperglycemia decreases lower esophageal sphincter pressure and the velocity of esophageal peristalsis (24).
Metabolic syndrome has important clinical and public health implications. In the United States, the unadjusted and age-adjusted prevalence of metabolic syndrome has been estimated to be 21.8% and 23.7%, respectively (25). In Japan, the prevalence of metabolic syndrome is 30.6% for men and 3.6% for women (26). In Korea, the prevalence of the metabolic syndrome was reported to be 13.5% for men and 15.0% for women (27). In the present study, the diagnostic components of metabolic syndrome (obesity, high triglyceride, low HDL cholesterol, high BP and elevated fasting glucose) based on NCEP criteria (7) were found to be significantly associated with RE. In metabolic syndrome, insulin resistance is an important pathogenic factor. Insulin resistance induces several metabolic changes, including hyperglycemia and dyslipidemia (28). Alternative mechanisms might include an association between metabolically active compounds and RE (29). These and other mechanisms by which obesity may affect risk for RE, such as growth factors, humoral factors like leptin, and hormonal factors like estrogen, were not examined in this study (30). A recent report showed components of the metabolic syndrome such as obesity, hyperglycemia, and hypertension are associated with the occurrence of RE in Japan (31). However, there was no report about the treatment of metabolic syndrome and improvement of RE until now.
Potential limitations still remain in our study. In this study, people who attended annual medical check-ups were enrolled for the assessment of RE. Therefore, the results of this study were similar, but not identical to those of population-based studies. We could not evaluate the reflux symptom such as heartburn, regurgitation, dyspepsia, epigastric pain, belching, and non-cardiac chest pain because of the retrospective study. In addition, we did not assess physical data such as waist-to-hip ratio, so we could not elucidate the prevalence of metabolic syndrome in our study population. This also prevented us from making inferences regarding the causality between metabolic syndrome and RE. However, this is first study to show the possibility that reflux esophagitis may be part of the disease spectrum of metabolic syndrome in a large sample of the Korean population.
In conclusion, the prevalence of RE in healthy subjects who had a routine check-up was 10.5% in Korea, and nearly approaches that reported in Western countries. Significant correlations between RE and obesity, high triglyceride, low HDL cholesterol, high BP, and elevated fasting glucose levels suggested that RE might be part of the disease spectrum of metabolic syndrome. Further studies are needed to evaluate the relationship between RE and metabolic syndrome.
Figures and Tables
Fig. 2
Prevalence of reflux esophagitis in each age group. The prevalence of reflux esophagitis was similar in each age group between both sexes.

Table 2
A comparison of clinical characteristics between subjects with reflux esophagitis and those without

*Data on Helicobacter pylori infection were unavailable for 224 (35.1%) subjects with reflux esophagitis and for 2,886 (53.0%) subjects without reflux esophagitis; †Data on high blood pressure were unavailable for 3 (0.5%) subjects with reflux esophagitis and for 33 (0.6%) subjects without reflux esophagitis.
BMI, body mass index; high blood pressure, ≥130/85 mmHg or documented use of antihypertensive therapy.
LDL, low-density lipoprotein; HDL, high-density lipoprotein.
References
1. Inamori M, Togawa J, Nagase H, Abe Y, Umezawa T, Nakajima A, Saito T, Ueno N, Tanaka K, Sekihara H, Kaifu H, Tsuboi H, Kayama H, Tominaga S, Nagura H. Clinical characteristics of Japanese reflux esophagitis patients as determined by Los Angeles classification. J Gastroenterol Hepatol. 2003. 18:172–176.

2. Goh KL, Chang CS, Fock KM, Ke M, Park HJ, Lam SK. Gastrooesophageal reflux disease in Asia. J Gastroenterol Hepatol. 2000. 15:230–238.

3. Furukawa N, Iwakiri R, Koyama T, Okamoto K, Yoshida T, Kashiwagi Y, Ohyama T, Noda T, Sakata H, Fujimoto K. Proportion of reflux esophagitis in 6010 Japanese adults: prospective evaluation by endoscopy. J Gastroenterol. 1999. 34:441–444.

4. Yeh C, Hsu CT, Ho AS, Sampliner RE, Fass R. Erosive esophagitis and Barrett's esophagus in Taiwan: a higher frequency than expected. Dig Dis Sci. 1997. 42:702–706.
5. Lee SJ, Song CW, Jeen YT, Chun HJ, Lee HS, Um SH, Lee SW, Choi JH, Kim CD, Ryu HS, Hyun JH. Prevalence of endoscopic reflux esophagitis among Koreans. J Gastroenterol Hepatol. 2001. 16:373–376.

6. Yeom JS, Park HJ, Cho JS, Lee SI, Park IS. Reflux esophagitis and its relationship to hiatal hernia. J Korean Med Sci. 1999. 14:253–256.
7. Miranda PJ, DeFronzo RA, Califf RM, Guyton JR. Metabolic syndrome: definition, pathophysiology, and mechanisms. Am Heart J. 2005. 149:33–45.

8. Hongo M. Minimal changes in reflux esophagitis: red ones and white ones. J Gastroenterol. 2006. 41:95–99.

9. Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, Gemmen E, Shah S, Avdic A, Rubin R. The burden of selected digestive diseases in the United States. Gastroenterology. 2002. 122:1500–1511.

10. Hampel H, Abraham NS, El-Serag HB. Meta-analysis: obesity and the risk for gastroesophageal reflux disease and its complications. Ann Intern Med. 2005. 143:199–211.

11. Kim Y, Suh YK, Choi H. BMI and metabolic disorders in South Korean adults: 1998 Korea National Health and Nutrition Survey. Obes Res. 2004. 12:445–453.

13. Nilsson M, Lundegardh G, Carling L, Ye W, Lagergren J. Body mass and reflux oesophagitis: an oestrogen-dependent association? Scand J Gastroenterol. 2002. 37:626–630.

14. Nilsson M, Johnsen R, Ye W, Hveem K, Lagergren J. Obesity and estrogen as risk factors for gastroesophageal reflux symptoms. JAMA. 2003. 290:66–72.

15. Gudlaugsdottir S, Verschuren W, Dees J, Stijnen T, Wilson J. Hypertension is frequently present in patients with reflux esophagitis or Barrett's esophagus but not in those with non-ulcer dyspepsia. Eur J Intern Med. 2002. 13:369–375.

16. Pilotto A, Franceschi M, Leandro G, Scarcelli C, D'Ambrosio LP, Seripa D, Perri F, Niro V, Paris F, Andriulli A, Di Mario F. Clinical features of reflux esophagitis in older people: a study of 840 consecutive patients. J Am Geriatr Soc. 2006. 54:1537–1542.

17. Lind T, Havelund T, Carlsson R, Anker-Hansen O, Glise H, Hernqvist H, Junghard O, Lauritsen K, Lundell L, Pedersen SA, Stubberod A. Heartburn without oesophagitis: efficacy of omeprazole therapy and features determining therapeutic response. Scand J Gastroenterol. 1997. 32:974–979.

18. Behar J, Biancani P, Sheahan DG. Evaluation of esophageal tests in the diagnosis of reflux esophagitis. Gastroenterology. 1976. 71:9–15.

19. Hongo M, Traube M, McAllister RG Jr, McCallum RW. Effects of nifedipine on esophageal motor function in humans: correlation with plasma nifedipine concentration. Gastroenterology. 1984. 86:8–12.

20. Nishida T, Tsuji S, Tsujii M, Arimitsu S, Sato T, Haruna Y, Miyamoto T, Kanda T, Kawano S, Hori M. Gastroesophageal reflux disease related to diabetes: analysis of 241 cases with type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004. 19:258–265.

21. Kinekawa F, Kubo F, Matsuda K, Inoue H, Kuriyama S. Gastroesophageal reflux disease in diabetic patients. Nippon Rinsho. 2004. 62:1546–1552.
22. Lluch I, Ascaso JF, Mora F, Minguez M, Pena A, Hernandez A, Benages A. Gastroesophageal reflux in diabetes mellitus. Am J Gastroenterol. 1999. 94:919–924.

23. Rayner CK, Samsom M, Jones KL, Horowitz M. Relationships of upper gastrointestinal motor and sensory function with glycemic control. Diabetes Care. 2001. 24:371–381.

24. De Boer SY, Masclee AA, Lam WF, Lamers CB. Effect of acute hyperglycemia on esophageal motility and lower esophageal sphincter pressure in humans. Gastroenterology. 1992. 103:775–780.

25. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002. 287:356–359.

26. Miyatake N, Wada J, Kawasaki Y, Nishii K, Makino H, Numata T. Relationship between metabolic syndrome and cigarette smoking in the Japanese population. Intern Med. 2006. 45:1039–1043.

27. Park HS, Lee SY, Kim SM, Han JH, Kim DJ. Prevalence of the metabolic syndrome among Korean adults according to the criteria of the International Diabetes Federation. Diabetes Care. 2006. 29:933–934.

28. Wassink AM, Olijhoek JK, Visseren FL. The metabolic syndrome: metabolic changes with vascular consequences. Eur J Clin Invest. 2007. 37:8–17.

29. Corley DA, Kubo A. Body mass index and gastroesophageal reflux disease: a systematic review and meta-analysis. Am J Gastroenterol. 2006. 101:2619–2628.

30. El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and reflux esophagitis. Am J Gastroenterol. 2005. 100:1243–1250.
31. Moki F, Kusano M, Mizuide M, Shimoyama Y, Kawamura O, Takagi H, Imai T, Mori M. Association between reflux esophagitis and features of the metabolic syndrome in Japan. Aliment Pharmacol Ther. 2007. 26:1069–1075.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download