INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease that affects many organ systems. As a cardiac manifestation, overt myocarditis is uncommon and not considered in the standard diagnostic criteria for SLE. Lupus myocarditis may rarely be the initial presentation as a fatal complication of SLE. Therefore, there is little information on clinical manifestations and outcomes of myocarditis in SLE.
CASE REPORT
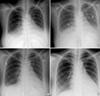
A 27-yr-old female patient was admitted due to dyspnea and orthopnea for 2 weeks. She gave birth to a baby 3 months previously. Clinical examination revealed bilateral pulmonary rales and pretibial pitting edema. Chest radiography showed cardiomegaly, interstitial pulmonary edema, and pleural effusion (Fig. 1A). Echocardiogram showed enlarged left atrium (LA; 45 mm) and left ventricular end-diastolic dimension/end-systolic dimension (LVEDD/LVESD; 57/49 mm), global hypokinesia of LV with decreased ejection fraction (LVEF; 30%), a small amount of pericardial effusion (LV anterior wall; 0.3 cm, right ventricular posterior wall; 1.3 cm), and mild pulmonary hypertension (right ventricular systolic pressure, RVSP; 47 mmHg) (Fig. 2A). We considered the diagnosis of peripartum cardiomyopathy at first because the patient never had a history of taking medicine or infection recently. The serelogic tests for human immunodeficiency virus, hepatitis B and C, and other viruses were also negative. We treated the patient in the customary manner with salt restriction, digitalis, angiotensin-converting enzyme inhibitor, and high dose of diuretics (intravenous furosemide; 20-80 mg/day, oral spironolactone; 50-200 mg/day). Even though we performed intensive heart failure therapy for 10 days, the symptoms were aggravated and interstitial pulmonary edema and pleural effusion were still remained on chest radiography (Fig. 1B). At that time, echocardiogram also showed increased LA size (45 mm → 51 mm) and severe pulmonary hypertension (RVSP; 47 mmHg → 61 mmHg) compared with the previous evaluation. So, we reviewed all laboratory findings and checked autoantibodies and complement level of the patient. Abnormal findings were as follows: anemia (hemoglobin; 8.8 g/dL), increased erythrocyte sedimentation rate (ESR; 33 mm/hr), proteinuria, hypoalbuminemia (2.5 g/dL), low C3 (30 mg/dL, normal; 86-184 mg/dL) and C4 level (5 mg/dL, normal; 20-58 mg/dL), positive ANA (1:640), positive anti-dsDNA (90.0 IU/m:, normal; <7 IU/m:), and positive anti-La antibody. We could make a diagnosis of SLE with acute myocarditis based on the evidence of the results. Intravenous methylprednisolone (1 g/day) for 5 days was given and followed by oral prednisolone (60 mg/day). The symptoms were dramatically improved and pulmonary edema was decreased on chest radiography in 48 hr after corticosteroid therapy (Fig. 1C). In 2 weeks after corticosteroid therapy, pulmonary edema and pleural effusion disappeared on chest radiography (Fig. 1D). Echocardiogram also showed much improved LVEF (55%), cardiac chamber size (LVEDD/LVESD; 55/37 mm, LA; 37 mm), and RVSP (30 mmHg) (Fig. 2B). We could also confirm lupus nephritis with proteinuria and findings of renal biopsy. Histologic findings showed diffuse glomerulonephritis with an active necrotizing lesion (WHO class IVB). Additional intravenous cyclophosphamide was given in a dose of 1.0 g for 1 day. The patient was maintained with a tapered prednisolone dose (40 mg/day) since then and will be treated with 12 courses of IV cyclophosphamide therapy.
DISCUSSION
In clinical studies, myocarditis has been identified in about 9% of patients with SLE (1). In our case, we could make a diagnosis as SLE based on the 1997 revised American College of Rheumatology (ACR) criteria for classification of SLE (2) such as photosensitivity, serositis (pericarditis), proteinuria, hemolytic anemia, lymphopenia, positive anti-dsDNA, and positive ANA. Most myocarditis in SLE is asymptomatic but may be manifest with fever, dyspnea, palpitation, and nonexertional chest pain (1). The gold standard of diagnosing myocarditis in SLE remains endomyocardial biopsy (3). However, endomyocardial biopsy is an invasive procedure and its diagnostic yield is very low at 10-20%. Therefore, the diagnosis of myocarditis in SLE depends largely on the clinical suspicion and echocardiographic findings. Echocardiographic findings in lupus myocarditis include decreased ejection fraction, increased chamber size, prolonged isovolumic relaxation time, decreased diastolic descent rate of the anterior mitral leaflet, decreased ratio of mean systolic velocity to mean diastolic velocity in the left ventricular posterior wall, decreased deceleration of early diastolic flow velocity and reduced E/A ratio, and atrial ejection force (3, 4). Our patient met those criteria. Echocardiography revealed enlarged LA (45 mm) and LVEDD/LVESD (57/49 mm), global hypokinesia of LV with decreased EF (30%), a small amount of pericardial effusion, and mild pulmonary hypertension (RVSP; 47 mmHg). Acute lupus myocarditis requires urgent clinical attention. Current treatment strategies are based on clinical experience rather than randomized trials. Treatment of lupus myocarditis should include an angiotensin-converting enzyme inhibitor and corticosteroids (5). A high dose of intravenous corticosteroid therapy (e.g., methyl-prednisolone pulses of 1.0 g/day for 5 days) is generally used, followed by an oral preparation (e.g., prednisolone; 1 mg/kg/day) for 1 to 2 weeks. Immunosuppressive agents like azathioprine (6) or cyclophosphamide (7), and combination of corticosteroids may also be beneficial in the treatment of lupus myocarditis. Additionally, there are some case reports on the beneficial use of intravenous immunoglobulin (IVIG) in lupus myocarditis (8). In our case, the patient showed dramatic resolution of refractory heart failure within 2 days after high-dose corticosteroid therapy.
The present case implicates that lupus myocarditis needs to be considered in refractory heart failure to massive conventional therapy, especially in young woman, and early treatment with corticosteroids, with or without immunosuppressive agents, may result in good outcomes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download