Abstract
Graves' disease (GD) is generally presented by thyrotoxicosis with hyperthyroidism, and it is an organ-specific autoimmune disease induced by thyroid-stimulating hormone receptor autoantibodies. However, among diverse etiologies, viral infections have been suggested to trigger or to be involved in the pathogenesis of GD. Hantaan virus infection causing hemorrhagic fever with renal syndrome (HFRS) is common in South Korea and its pathogenesis is suggested to be an immunologic mechanism. We have experienced a patient who was diagnosed as HFRS with thyrotoxicosis. So we herein report the case as GD combined with the hantaan virus infection.
Graves' disease (GD) is an autoimmune disease presented by hyperthyroidism. Although the etiology and pathogenesis of GD are still not clear, environmental factors including virus infection are suggested to be involved (1). Among these, retrovirus, enterovirus (especially Coxsackie B virus), and influenza B virus were reported with serologic evidence (2-4). However, there has been no report on hantaan virus infection with GD. The hantaan virus is common in South Korea and can cause hemorrhagic fever with renal syndrome (HFRS) (5).
We herein describe the first case of HFRS with GD associated with hantaan virus infection.
A 19-yr-old man was admitted to Chonbuk National University Hospital, South Korea with fever, dyspnea, palpitation, sweating, and drowsiness. On admission his vital signs were blood pressure 140/90 mmHg, heart rate 136/min, body temperature 38.1℃, and respiratory rate 45/min. On physical examination, the patient appeared acutely ill and presented with petechiae in the throat, erythematous rash on the body, conjunctival suffusion without eyeball protrusion, mild non-tender swelling of the neck area with thrill, and abdominal tenderness with hepatosplenomegaly. Before admission, he had no medical problems and had no family history of medical disease. Recently, his parents observed the patient's trend of weight loss and irritability, but he had no complaint of his health status. Laboratory findings are shown in Table 1. The patient was treated with fluid therapy in balance with urine output, empirical antibiotics, methimazole, and non-selective beta blocker. On day 4, hypothermia and hypotension occurred, and on day 6, oliguria developed. However, the patient was tolerable and so supportive fluid therapy was continued with the monitoring of urine amount. Thyroid ultrasonography and scan were also performed (Fig. 1), which showed findings compatible with hyperthyroidism. On day 14, the patient was stabilized in terms of physical and laboratory findings (Fig. 2, Table 1) and was discharged with complete recovery on day 21. Before discharge, the titer of hantaan virus and thyroid function test including thyrotropin binding inhibitory immunoglobulin (TBII) were re-checked (Table 1) and the results strongly showed that the diagnosis of GD and hantaan virus infection and treatment targeting to these diseases had been reasonable.
GD is an organ-specific autoimmune disease and characterized by autoimmune hyperthyroidism caused by thyroid-stimulating hormone (TSH) receptor autoantibody and several cytokines such as interleukin-1 (IL-1), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ) were reported to be involved in the activation of TSH receptor (1, 6). In addition, both genetic and environmental factors are also believed to contribute to the development of GD; however, etiologies and pathogenesis remain unclear (6). Recently, several studies have suggested that viral infection may be involved in the pathogenesis of GD (2-4). Evidence that infection might be related with GD has been shown by epidemiology, serology, and molecular methods. Proposed possible mechanisms are molecular mimicry between the TSH receptor and viral antigen, superantigenic stimulation of autoreactive lymphocytes (T cell activation), anti-idiotypic antibodies reactive with the TSH receptor (6, 7), formation of immune complexes or induction of immune response (8), and induction of classs II major histocompatibility complex (MHC) antigen on thyrocytes (9, 10). However, hantaan virus infection causing HFRS has not been reported as a triggering factor of GD.
Hantaan viruses are found wherever rodents of the family Muridae are present and can cause HFRS through aerosols of infectious rodent urine (5). The pathogenesis of hantaan virus infection is still unclear, however, an immunopathologic process has been suggested to be one of the involved mechanisms. Hantaan virus infection also causes clinical representations by diverse mediators such as TNF-α, and IFN-γ, IL-4, and IL-6 (11). In this respect, as with other known viruses, hantaan virus can also be involved in the pathogenesis of GD, although the exact mechanism and mediating factors are not known. In this case, the patient was diagnosed as HFRS due to hantaan virus infection by laboratory findings and clinical course.
In summary, we herein report a case of HFRS caused by hantaan virus infection presenting simultaneously with GD. However, further research is needed to find etiologic factors in induction of GD with a direct approach using infecting organism isolation to determine whether it was triggered by a hantaan virus infection. Also, a retrospective analysis of HFRS or GD about combined diseases or prospective investigation of GD with hantaan virus infection or HFRS with thyrotoxicosis should be performed to demonstrate the causal relationship between hantaan virus infection and GD.
Figures and Tables
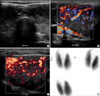 | Fig. 1Thyroid ultrasonography using 7.5 MHz probe and 99m-Tc (technecium) thyroid scan of the patient. (A) The anteroposterior diameter of the isthmus was increased to 6 mm. (B) The right lobe showed heterogenic echogenecity with increased vascularity and enlarged size. (C) The left lobe showed thyroid inferno and both lobes showed the same findings. (D) Both lobes showed increased trapping without a nodular region. |
 | Fig. 2Vital signs during hospitalization. (A) Body temperature change. (B) Systolic (Sys) and diastolic (Dia) blood pressure (BP) and heart rate (HR) changes. (C) Urine output change. |
Table 1
Laboratory findings of the patient during admission

TBII: thyrotropin binding inhibitory immunoglobulin; tested by thyrotropin receptor autoantibodies kit using 125I-labelled TSH produced from RSR Ltd., Cardiff, U.K. Hantaan virus; tested by Genedia Hantadia® (Greencross, South Korea)
AST, aspartate aminotransferase; ALT, alanine aminotransferase; Bun/Cr, blood urea nitrogen/creatinine; CRP, c-reactive protein; TSH, thyroid stimulating hormone.
References
2. Sander DM, Wolfsheimer K, Gallaher WR, Fermin CD, Haislip AM, Garry RF. Seroreactivity to A-type retrovirus proteins in a subset of cats with hyperthyroidism. Microsc Res Tech. 2005. 68:235–238.

3. Pichler R, Maschek W, Hatzl-Griesenhofer M, Huber H, Luger C, Binder L, Mittermayer H. Enterovirus infection-a possible trigger for Graves' disease? Wien Klin Wochenschr. 2001. 113:204–207.
4. Wolf MW, Misaki T, Bech K, Tvede M, Silva JE, Ingbar SH. Immunoglobulins of patients recovering from Yersinia enterocolitica infections exhibit Graves' disease-like activity in human thyroid membranes. Thyroid. 1991. 1:315–320.
5. Peters CJ, Khan AS. Hantavirus pulmonary syndrome: the new American hemorrhagic fever. Clin Infect Dis. 2002. 34:1224–1231.

7. Davies TF, Martin A, Concepcion ES, Graves P, Cohen L, Ben-Nun A. Evidence of limited variability of antigen receptors on intrathyroidal T cells in autoimmune thyroid disease. N Engl J Med. 1991. 25:238–244.

8. Jordan SC, Buckingham B, Sakai R, Olson D. Studies of immune-complex glomerulonephritis mediated by human thyroglobulin. N Engl J Med. 1981. 304:1212–1215.

9. Davies TF, Piccinini LA. Intrathyroidal MHC class II antigen expression and thyroid autoimmunity. Endocrinol Metab Clin North Am. 1987. 16:247–268.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download