INTRODUCTION
Hyperaldosteronism as a secondary cause of hypertension has been well known. However, the metabolic effects of this condition and its association with parathyroid hormone in causing a pro-inflammatory state in the body have been recognized only recently (1, 2). Aldosterone-salt treatment (AldoST) in rats, similarly chronic mineralocorticoid excess states in humans, is known to cause a fall in plasma concentrations of ionized Ca2+ and Mg2+ by increased urinary and fecal excretion of these divalent cations (3, 4). The resulting secondary hyperparathyroidism is thought to play a significant role in the patho-physiology associated with this condition.
CASE REPORT
A 28-yr-old college lecturer from interior southern India, had complaints of polyuria since November 2001. Polyuria was noticed only in recumbent position irrespective of the time of the day. She first consulted an urologist, who diagnosed nephrolithiasis for which she underwent extracorporeal short wave Lithotripsy (ESWL). At that time she was detected to have a blood pressure of 170/90 mmHg and also noted to have spasms of the upper limb while recording the blood pressure. She was examined by the local physician who diagnosed hypertension and started her on amlodipine. Blood chemistries done at that time including calcium and phosphorus were normal and potassium was 3.56 mM/L (3.5-5). Her urinary symptoms continued to persist despite treatment. In 2003 she became pregnant and carried through the pregnancy well until 32 weeks. Pregnancy had to be terminated with a caesarean section when it became increasingly difficult to control her blood pressure. After delivery she started having severe cramps in both upper and lower limbs. An endocrinology consultation was sought in 2004 for recurrent cramps, a thorough work up was done and results are shown in Table 1.
Since no obvious cause for hypocalcemia was found, an intrinsic calcium absorption defect was suspected and patient was advised six grams of calcium orally, without any symptomatic improvement. In the subsequent months, atenolol, losartan and prazosin were all added, at different times, in order to control the blood pressure.
She was first seen in our institution in April 2005 with the above described complaints in the urology department. She had a significant weight loss of 12 kg in the past year. She had never smoked cigarettes or consumed alcohol. None of the family members were hypertensive or had adrenal or other endocrine tumors.
Her physical examination revealed a blood pressure of 150/ 90 mmHg, without any postural variation. Carpal spasm was noticed when blood pressure was being taken. Pulse rate was 80/min and respiratory rate was 18/min. She was 165 cm tall and weighed 64 kg. Systemic examination was unremarkable. A complete urological work up including plain abdominal radiography, intra venous pyelography, micturating cystourethrogram were all done which failed to explain her symptoms. Simultaneously she was being worked up for secondary causes of hypertension. The results of the investigations are shown in Table 2.
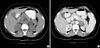
Sonography of the abdomen had revealed multiple hyperechoeic foci with shadowing in the right kidney, mild hepatomegaly measuring 15 cm with normal echotexture and a well defined hypoechoeic lesion measuring about 3×3×3 cm in the left suprarenal region. Bilateral renal Doppler showed few renal indices in the upper limit of the normal. Hence renal artery stenosis was ruled out with renal angiogram. Serum aldosterone in standing position was elevated at 318.94 ng/L (5-41). Electrocardiography showed left ventricular hypertrophy with strain pattern while 2D Echocardiography revealed slight enlargement of the left ventricular chamber with a normal ejection fraction and mild Mitral regurgitation. A computed tomography scan of abdomen confirmed a well defined exophytic mass lesion (3×2.8×2.8 cm) from the left adrenal mass (Fig. 1).
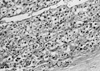
Patient was optimized for surgery with multiple antihy-pertensive drugs including spironolactone, atenolol and amlodipine with which stable blood pressure control was achieved. She underwent left adrenalectomy under general anesthesia and the exophytic mass was resected. Patient tolerated the procedure well and made a good post operative recovery. Operative specimen and histopathology was consistent with adrenal adenoma (Fig. 2, 3).
Her blood pressure started normalizing and her anti-hypertensive medications were gradually tapered. At follow-up after 6 weeks patient was off antihypertensive medication and blood pressure was normal. Her repeat biochemistries revealed potassium of 4.3 meq/L and calcium of 9.7 mg/dL (8.7-10.2). She did not have cramps and had regained 12 kg. At one year follow-up, she has regained her pre-morbid weight and continues to be normotensive and symptom free.
DISCUSSION
The syndrome of primary aldosteronism, first described by Conn (5) in 1955, is characterized by hypertension, hypokalemia, suppressed plasma rennin activity, and increased aldosterone excretion. Hypertension is caused by excessive sodium retention and body fluid expansion mediated by the aldosteronism. Hypokalemia results from the urinary loss of potassium, because it is exchanged for sodium in the distal renal tubule under the influence of aldosterone. Volume expansion and sodium retention cause inhibition of renal release of renin, which leads to low plasma renin activity. If hypokalemia is severe, it may cause muscle weakness, cramps, or even paralysis.
Our case is unique because this patient presented with almost entire constellation of symptoms and metabolic effects described in aldosterone excess state. Although the diagnosis remained obscure for more than two years until it finally unveiled itself. Association of hyperaldosteronism with renal calculi is known and has been reported earlier (6). Postural variation in urine output was quite characteristic in our patient. Previous study has described a small subgroup of primary aldosteronism, named aldosterone-producing reninresponsive adenoma (AP-RA), have significant postural variation in concentration of aldosterone levels when subjected to upright posture. This might partially explain her symptom of supine polyuria (7).
Urinary calcium excretion was normal in our patient, but 24 hr urinary phosphate excretion was high. Increased urinary calcium excretion in PA has been noted only at an elevated sodium intake (8). The probable mechanism is thought to be related to expansion of the extravascular space, resulting in decreased proximal tubular reabsorption and thereby increased distal delivery of Na+, Mg2+, Ca2+, with the mineralocorticoid promoting distal tubular Na+ reabsorption without retarding magnesium or calcium excretion (3). While increased phosphate excretion could be due to ensuing secondary hyperparathyroidism due to hypocalcemia. However, sodium intake was not standardized while measuring the 24 hr urinary calcium excretion in our patient.
In one study, patients with PA were detected to have significantly higher concentration of parathyroid hormone (PTH) compared to both normal and essential hypertensive subjects, consistent with our case (2). Severe weight loss due to hyperaldosteronism has been reported earlier (9, 10). Aldosteronism, by causing secondary hyperparathyroidism, is thought to cause a pro-inflammatory state characterized by oxidative stress, immune cell activation, vascular remodeling by invading inflammatory cells, and bone loss (1). This effect has been studied in the setting of secondary hyperaldosteronism associated with cardiac failure and cachexia (11). We feel that further insight needs to be shed into this effect of hyperaldosteronism and the mechanism by which it causes weight loss needs to be elucidated.
Primary hyperaldosteronism represents one of the few treatable causes of hypertension. In addition to hypertension which is often difficult to control it may manifest through symptoms and signs due to its various metabolic effects. A high index of suspicion and a systematic approach is instrumental in identifying and proving the diagnosis. Patients in whom aldosteronomas are identified should be subjected to surgery which can be curative.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download