INTRODUCTION
Pyoderma gangranosum is an idiopathic, inflammatory, ulcerative disease of undetermined cause. In addition to local wound care, management often includes the use of systemic corticosteroids or systemically administered immunomodulatory agent. Pyoderma gangranosum typically does not require surgical management.
We report a patient who developed pyoderma gangranosum in the penis with invasion of the distal urethra. The patient was treated with prednisolone and thalidomide, followed by a reconstructive surgical repair using a scrotal island flap.
CASE REPORT
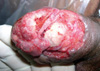
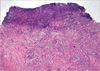
A previously healthy 59-yr-old man presented with a severely painful ulceration in the distal penile shaft and glans of the penis that developed about 1 month earlier. The lesion was characterized by an irregular margin and a dirty irregular base with oozing. The patient denied genital trauma and did not have extramarital sex during the preceding 6 months. There were painful purulent ulcerations on the distal dorsal penile shaft and glans of the penis with meatal stenosis and multiple urethrocutaneous fistulas (Fig. 1). There was neither inguinal nor femoral adenopathy and physical examination was otherwise normal. Thorough evaluation of sexually transmitted diseases gave no abnormal findings. Moreover, the following investigations were normal and revealed no evidence of any associated disease: complete blood counts, renal and liver functions, rheumatoid factor, antinuclear antibodies, antineutrophilic cytoplasmic antibodies, anti-dsDNA, colonoscopy, chest radiography and magnetic resonance imaging of the pelvis. Repeated swabs yielded coagulase-negative staphylococcus. Skin histology was consistent with pyoderma gangrenosum (Fig. 2).
He was admitted for aggressive treatment consisting of prednisolone (5 mg 4 times daily), mesalazine (500 mg 3 times daily), potassium permanganate baths and suprapubic cystostomy. Systemic ciprofloxacin, kanamycin and metronidazole were also administered. However, 3 days later, there was still purulent discharge and the glans appeared to be at risk of sloughing off. Prednisolone was increased to 10 mg 4 times daily. This regimen was well tolerated for 10 days with a marked improvement in the appearance of the ulcerations but with a penile deformity. The dose of prednisolone was progressively reduced and was discontinued over the next 6 weeks. Mesalazine was continued.
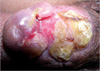
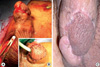
After medical treatment, the skin ulcerations improved with re-epithelialization. However, the penile deformity (dorsal curvature) aggravated, which was indicated for an early reconstructive operation (Fig. 3). The necrotic tissue was excised and the skin defects of the glans and distal shaft were repaired with a scrotal island flap. Urethral and suprapubic catheterization was done (Fig. 4A, B). During the perioperative period, he was administered prednisolone (5 mg 4 times daily) and mesalazine (500 mg 3 times daily) for the prevention of pathergy at the operative site. On the 8th postoperative day, the urethral Foley catheter was removed and these medications were stopped without subsequent recurrence of ulcerations. Micturition was normal. However, urethrocutaneous fistulas developed on the postoperative 20th day without recurrence of pyoderma gangranosum. These medications were maintained over several weeks with continuous suprapubic catheterization. All skin lesions were completely cured without recurrence of pyoderma gangrenosum 17 months after reconstructive surgery.
DISCUSSION
Pyoderma gangranosum is an uncommon skin disorder characterized by ulcerative lesions that most often affect the lower extremities but may involve the face, neck, scrotum or penis (1, 2). The etiology of pyoderma gangranosum is poorly understood, but approximately 50 percent of patients with pyoderma gangranosum have an associated systemic disorder, with inflammatory bowel disease being the most common (2). There have been less than 15 previous reports of pyoderma gangranosum involving the penis (3-7). A case of pyoderma gangrenosum involved in female genitalia was reported in Korea (8). Two reported cases of penile pyoderma gangranosum were associated with systemic chronic lymphocytic leukemia (9) and ulcerative colitis (10) respectively, but the others occurred without any associated diseases. Pyoderma gangranosum is a disease entity diagnosed when other causes of purulent ulcerations, such as STD, multi-system disease, necrotizing faciitis, cutaneous metastatic Crohn's disease, deep fungal infection, pemphigus vegetans, Fournier's gangrene, neoplastic conditions, erosive lichen planus, trauma and factitious damage have been excluded.
Early recognition is critical to avoid unnecessary or potentially harmful interventions. The extension of lesions in response to trauma or surgical debridement, which is termed pathergy, is a hallmark of pyoderma gangranosum (11). Local wound care is essential to ensuring a suitable wound environment for healing, and prevention and treatment of secondary bacterial infection. Thus, ulcers should be gently cleansed daily with saline lavage, and sometimes diluted potassium permanganate solution (1:20,000) is helpful. Nonadherent dressing is most suitable for the ulcerations because it can be easily changed and can reduce the risk for trauma (12). Therapeutic options for penile pyoderma gangranosum include topical or systemic corticosteroids. Additionally, other agents have been used in combination with steroids or as monotherapy. Minocycline, thalidomide, cyclophosphamide or 5-aminosalicylic acid have been reported to be effective (1, 7). Since the ulcerations were extremely severe, the patient was at high risk for sloughing-off of the glans, thus warranting aggressive medical therapy and requiring a subsequent surgical repair. The regimen of prednisolone (40 mg daily) and mesalazine was successfully administered. The ulceration markedly improved, but a penile deformity developed because of contraction of the scar. This is the reason that we attempted to operate the wound immediately. The perioperative regimen was the same as the prior one. Although urethrocutaneous fistulas developed and the discharge aggravated during the early postoperative period, the wound improved after urinary diversion and continuation of the medications. There was no evidence of recurrence of pyoderma gangranosum or any complications for 17 months.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download