Abstract
The aim of this study was to develop a new simple method for measuring the vaporized volume and to evaluate the outcome of high-power potassium-titanyl-phosphate (KTP) photoselective laser vaporization. A total of 65 patients, with a mean age of 67.7 yr (range 53 to 85), were included in the primary analysis. The vaporized volume was calculated as the pre-operative volume minus the immediate post-operative volume plus the volume of the defect. For all patients, the subjective and objective parameters improved significantly after surgery. Six and 12 months after surgery, the group with a smaller vaporized volume (<15 g) had a lower reduction of the mean International Prostate Symptom Score (P=0.006 and P=0.004) and quality of life index (P=0.006 and P=0.004) when compared to the group with a greater vaporized volume (≥15 g). There were no differences in the change of the maximum flow rate and post-void residual based on the vaporized volume. Our findings suggest that the subjective improvement, after a high-power KTP laser vaporization, may be dependent on the vaporized volume obtained after the procedure.
Transurethral resection of the prostate (TURP) is considered the standard treatment for men with benign prostatic hyperplasia (BPH) (1). However, this procedure is associated with significant morbidity. In order to minimize the perioperative morbidity of TURP procedures, various minimally invasive alternatives have been introduced into clinical practice. The high-power potassium titanyl phosphate (KTP) photoselective laser vaporization of the prostate (PVP) is emerging as a popular technology. Vaporization of the prostate with an 80 W KTP laser combines the tissue-debulking properties of the TURP with the desirable hemostatic properties of laser procedures (2).
The complete resection of the prostate is the goal of TURP procedures. However, a considerable volume of the prostate may remain despite endoscopic appearances, after complete resection of an adenoma (3). Chen et al. (4) reported that a better clinical result after a TURP correlated significantly with the completeness of the resection of the obstructing adenoma. The impact of the vaporized volume after a PVP has not been systematically investigated. In the present study, we estimated the volume of the prostate that were vaporized after a PVP and determined their impact on the clinical outcomes in men with lower urinary tract symptoms/BPH.
Approval for this study was obtained from the Institutional Review Board of the Seoul National University Hospital. The clinical records of 65 men, with a mean age of 67.7 yr (range 53 to 85), who underwent PVP were retrospectively reviewed. Inclusion and exclusion criteria, for the operative technique, have been reported previously (5). Patients were evaluated before the PVP by the International Prostate Symptom Score (IPSS), quality of life (QOL) index, maximum flow rate (Qmax), post-void residual (PVR), prostate volume, and serum prostate-specific antigen (PSA) levels. In patients with elevated PSA or a digital examination suspicious of prostate cancer, prostate biopsy was performed to document the absence of prostate cancer. If biopsies were negative for cancer, the patients were included in this study.
To ensure that the vaporized volume calculated was accurate, pilot testing was conducted on 10 patients who underwent TURP procedures (n=9) or an open prostatectomy (n=1). Immediately before and after the surgery, in the operating room, the prostate volume was measured by transrectal ultrasonography (TRUS) using the prolate ellipse formula (6). The prostate tissue collected at resection was weighed and the value was then recorded. The calculated volume of the resected tissue was determined by the formula: calculated resected volume=pre-operative volume-post-operative volume+volume of the defect. The defected volume was estimated using the prostate volume calculation. We confirmed that there was a significant correlation between the actual weight of the surgical specimen and the estimated volume of the resected tissue (r=0.970, P<0.001). Then, the vaporized volume was estimated in 65 patients who underwent a PVP using the same method and the same formula (Fig. 1). All TRUS were performed by a single radiologist.
Follow-up was carried out at 1, 3, 6, and 12 months. At each visit, the IPSS was recorded; in addition, Qmax and PVR were recorded at 3, 6, and 12 months postoperatively. The prostate volume was measured at 6 months postoperatively. The primary outcome measure was the change in subjective symptoms. The Qmax and PVR were secondary outcome measures.
For the statistical analysis, the patients were stratified into two groups according to the median value at the cutoff of 14.8 g for vaporized volume; the vaporized volume was classified as <15 g (n=32) and ≥15 g (n=33). A comparison between the groups was conducted using the Student's t test for the continuous data and the Armitage test for the categorical data. A 5% level of significance was adopted for all statistical testing, and all statistical tests were two-sided. Statistical analysis was performed using a commercially available data analysis program, Statistical Package for Social Sciences, version 13.0 (SPSS, Chicago, IL, U.S.A.).
The baseline patient characteristics are shown in Table 1. The prostate volume were greater in the group with more vaporized volume (P<0.001). The patients with a greater vaporized volume had a higher baseline PVR (P=0.001). The PVP procedure was slightly faster in the group with a lower vaporized volume when compared to those with a greater vaporized volume (P=0.019). The total mean energy delivery for the two groups was 165.6 and 204.0 kJ, for the lower and greater volume vaporization, respectively (P=0.020). No statistical differences were observed regarding the other parameters evaluated.
No severe intraoperative complication was observed. Mild hematuria was identified in 18 patients (27.7%), which did not require medical attention. One patient (1.5%) experienced clot retention postoperatively and had catheter successfully removed after 2 weeks. Postoperative transient urinary tract irritation including urgency was experienced by 2 patients (3.1%), which resolved without medical intervention. Acute epididymitis was seen after the surgery in 1 patients (1.5%) and a postoperative urethral stricture was observed in 1 (1.5%). No blood transfusions were required and no other perioperative complications were noted.
As the baseline prostate volume increased, the calculated vaporized volume increased (r=0.709, P<0.001). The increased values were observed at the time of the surgery (r=0.447, P<0.001) and the energy delivered increased (r=0.381, P=0.002) as the calculated vaporized volume increased (data not shown).
The one year outcome data are shown in Figs. 2, 3. In each group, an immediate and highly significant improvement of the subjective and objective outcomes was evident. After treatment, no statistical difference in the IPSS and the QOL index was observed between the groups at the early follow-up of three months; however, subjective improvements were significantly higher in the group with a greater vaporized volume (P<0.05) (Fig. 2). During the follow-up period, the degree of improvement of the Qmax was similar in both groups. There was no statistical difference in the PVR observed between the groups after three months of follow up; however, the rate of improvement in the PVR, in the group with a greater vaporized volume, was significantly higher compared to the patients with a lower vaporized volume (P<0.05) (Fig. 3).
Since the PVP creates a TUR-like cavity, its mechanism of action is similar to that of the traditional TURP. Sulser et al. (7) evaluated the pre-operative and post-operative prostate volume and found that the average prostate volume changed from 51.2 mL to 35.8 mL at three months after a PVP. Kumar (8) and Sarica et al. (9) reported a 51% and a 53% mean decrease in the prostate volume during a mean follow-up of 2.8 months and at 12 months in patients undergoing PVP. In the present study, the pre-operative prostate volume was 61.7±3.2 mL and at six months, the prostate volume decreased to 37.0±3.6 mL. These findings suggest that the PVP can result in a significant prostate volume reduction.
In a non-randomized prospective study, the early outcomes of PVP were found to be similar to those of TURP for relatively small prostates (10). The first randomized comparison of TURP with PVP also revealed that KTP laser vaporization was equivalent to TURP in terms of decreasing IPSS and increasing the Qmax, similar to the patients with relatively small prostates (TURP 33.2 mL vs. PVP 42.4 mL) (11). However, Hai and Malek found a 40% reduction in prostate volume three months following a PVP but subsequent measurements at 12 months showed an increase in the prostate volume (12). In a multicenter study, there was an overall 29% reduction in the prostate volume, and only a 17% reduction in the serum PSA level at three years after the procedure (13). Te et al. (13) suggested that the overall results achieved with a PVP are very positive and durable up to three years; however, the clinical outcome for smaller and larger prostates treated by PVP may be disproportionate. A recent prospective randomized study demonstrated that a significant difference in the IPSS, Qmax and PVR values was observed during the follow-up period, and the results favored the TURP procedure for prostates larger than 70 mL; the percentage volume reduction was significantly higher in the TURP group (14). The percent reduction of the prostate volume was significantly higher in the TURP group at both three (64.1% vs. 43.3%) and six months (62.9% vs. 40.5%) and the percent reduction of the serum PSA levels was also higher in the TURP group at three (43.7% vs. 29.3%) and six months (44.6% vs. 31.8%). Pfitzenmaier et al. found that there was a significant trend toward a higher re-operation rate after a PVP in men with larger prostates (15).
In ex vivo experiments, tissue ablation with the KTP laser was five times slower than TURP-like tissue resection (16). However, although tissue ablation with the KTP laser seems to be more time-consuming than standard tissue resection, the slower ablation performance of the KTP laser might be compensated by its better hemostatic performance. In fact, many investigators have suggested that the PVP is safe and effective, with durable results for men with large volume prostates (15, 17-19). In our series, the serum PSA and prostate volume at baseline, in the group with a greater vaporized volume, were higher than those with a smaller vaporized volume. Furthermore, the serum PSA and prostate volume remained higher in the group with a greater vaporized volume. Nevertheless, at 6 and 12 months after surgery, the group with a greater vaporized volume showed a greater reduction in the mean IPSS and QOL index, although the Qmax did not increase in parallel with the decrease in the IPSS after the PVP procedure. The time for the laser treatment was generally longer in patients with larger prostates. In addition, there was a relationship between the amount of tissue vaporization and the amount of energy delivered. These findings suggest that the PVP is an effective method for treating BPH caused by large prostates and that subjective improvement after PVP may be dependent on the volume vaporized. The determination of the appropriate treatment modality based on the size of the prostate and other factors remains the judgment of the treating surgeon.
Although sonographic estimates of prostate volume have been widely applied, prostate volume determined by TRUS is subject to inter-observer variation. For example, if a transducer is placed at different sites for the caudal end of the prostate, the prostate volume measured can be different. However, our findings were not altered by inter- or intra-observer variation because the same examiner used the same method for the same period of observation.
To our knowledge, our study is the first to estimate the volume of the prostate vaporized after a PVP and to determine their impact on the clinical outcomes. In the present study, we added the defected volume to measure the resected volume. We learned from our patients underwent TURP procedures that the calculated resected volume tends to be underestimated compared to the true resected volume. Thus, we tested new formula in this pilot study and found a very significant correlation between the actual weight of the surgical specimen and the estimated volume of the resected tissue (r=0.970, P<0.001).
In conclusion, the findings of this study suggest that the PVP is a safe and effective method for treating BPH caused by small or large prostates. In addition, the results suggest that the subjective improvements noted after PVP may be dependent on the volume of prostate tissue vaporized. However, the determination of the most appropriate treatment modality, based on the size of the prostate and other factors, remains the decision of the treating surgeon.
Figures and Tables
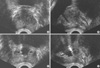 | Fig. 1Calculated vaporized volume was determined by the formula: vaporized volume=pre-operative volume-immediate post-operative volume+defected volume. (A) transverse view (preoperative). (B) sagittal view (preoperative). (C) transverse view (postoperative). (D) sagittal view (postoperative). Arrows indicate vaporized volume immediately after photoselective vaporization of the prostate. Calculated vaporized volume was 18.7 mL (50-37+5.7). True vaporized volume was not checkable. |
 | Fig. 2Change in clinical parameters according to vaporized volume. (A) International Prostate Symptom Score. (B) Quality of life index. White and black circles circle indicate the scores in the groups with the vaporized volume ≥15 g and <15 g at each month, respectively. Gray and black bars indicate the score changes in the groups with the vaporized volume ≥15 g and <15 g at each month, respectively. |
 | Fig. 3Change in clinical parameters according to vaporized volume. (A) maximum flow rate. (B) post-void residual. White and black circles circle indicate the scores in the groups with the vaporized volume ≥15 g and <15 g at each month, respectively. Gray and black bars indicate the score changes in the groups with the vaporized volume ≥15 g and <15 g at each month, respectively. |
References
1. Barber NJ, Muir GH. High-power KTP laser prostatectomy: the new challenge to transurethral resection of the prostate. Curr Opin Urol. 2004. 14:21–25.

2. Tan AH, Gilling PJ. Lasers in the treatment of benign prostatic hyperplasia: an update. Curr Opin Urol. 2005. 15:55–58.

3. Green JS, Bose P, Thomas DP, Thomas K, Clements R, Peeling WB, Bowsher WG. How complete is a transurethral resection of the prostate? Br J Urol. 1996. 77:398–400.
4. Chen SS, Hong JG, Hsiao YJ, Chang LS. The correlation between clinical outcome and residual prostatic weight ratio after transurethral resection of the prostate for benign prostatic hyperplasia. BJU Int. 2000. 85:79–82.

5. Paick JS, Um JM, Kwak C, Kim SW, Ku JH. Influence of bladder contractility on short-term outcomes of high-power potassium-titanylphosphate photoselective vaporization of the prostate. Urology. 2007. 69:859–863.

6. Littrup PJ, Williams CR, Egglin TK, Kane RA. Determination of prostate volume with transrectal US for cancer screening. Part II Accuracy of in vitro and in vivo techniques. Radiology. 1991. 179:49–53.

7. Sulser T, Reich O, Wyler S, Ruszat R, Casella R, Hofstetter A, Bachmann A. Photoselective KTP laser vaporization of the prostate: first experiences with 65 procedures. J Endourol. 2004. 18:976–981.

8. Kumar SM. Photoselective vaporization of the prostate: a volume reduction analysis in patients with lower urinary tract symptoms secondary to benign prostatic hyperplasia and carcinoma of the prostate. J Urol. 2005. 173:511–513.

9. Sarica K, Alkan E, Luleci H, Tasci AI. Photoselective vaporization of the enlarged prostate with KTP laser: long-term results in 240 patients. J Endourol. 2005. 19:1199–1202.

10. Bachmann A, Schurch L, Ruszat R, Wyler SF, Seifert HH, Muller A, Lehmann K, Sulser T. Photoselective vaporization (PVP) versus transurethral resection of the prostate (TURP): a prospective bi-centre study of perioperative morbidity and early functional outcome. Eur Urol. 2005. 48:965–971.

11. Bouchier-Hayes DM, Anderson P, Van Appledorn S, Bugeja P, Costello AJ. KTP laser versus transurethral resection: early results of a randomized trial. J Endourol. 2006. 20:580–585.

12. Hai MA, Malek RS. Photoselective vaporization of the prostate: initial experience with a new 80 W KTP laser for the treatment of benign prostatic hyperplasia. J Endourol. 2003. 17:93–96.

13. Te AE, Malloy TR, Stein BS, Ulchaker JC, Nseyo UO, Hai MA. Impact of prostate-specific antigen level and prostate volume as predictors of efficacy in photoselective vaporization prostatectomy: analysis and results of an ongoing prospective multicentre study at 3 years. BJU Int. 2006. 97:1229–1233.

14. Horasanli K, Silay MS, Altay B, Tanriverdi O, Sarica K, Miroglu C. Photoselective potassium titanyl phosphate (KTP) laser vaporization versus transurethral resection of the prostate for prostates larger than 70 mL: a short-term prospective randomized trial. Urology. 2008. 71:247–251.

15. Pfitzenmaier J, Gilfrich C, Pritsch M, Herrmann D, Buse S, Haferkamp A, Djakovic N, Pahernik S, Hohenfellner M. Vaporization of prostates of >or=80 mL using a potassium-titanyl-phosphate laser: midterm-results and comparison with prostates of <80 mL. BJU Int. 2008. 102:322–327.
16. Reich O, Bachmann A, Schneede P, Zaak D, Sulser T, Hofstetter A. Experimental comparison of high power (80 W) potassium titanyl phosphate laser vaporization and transurethral resection of the prostate. J Urol. 2004. 171(6 Pt 1):2502–2504.

17. Sandhu JS, Ng C, Vanderbrink BA, Egan C, Kaplan SA, Te AE. High-power potassium-titanyl-phosphate photoselective laser vaporization of prostate for treatment of benign prostatic hyperplasia in men with large prostates. Urology. 2004. 64:1155–1159.

18. Tugcu V, Tasci AI, Sahin S, Ordekci Y, Karakas OF, Zorluoglu F. Outcomes of 80 W KTP laser vaporization of the large prostate. Urol Int. 2007. 79:316–320.

19. Rajbabu K, Chandrasekara SK, Barber NJ, Walsh K, Muir GH. Photoselective vaporization of the prostate with the potassium-titanylphosphate laser in men with prostates of >100 mL. BJU Int. 2007. 100:593–598.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download