Abstract
The aim of this study was to evaluate the occurrence of genital mycoplasmas, especially Ureaplasma parvum and Ureaplasma urealyticum, in women with atypical squamous cells of undetermined significance (ASCUS), low grade squamous intraepithelial lesions (LSIL) and high grade squamous intraepithelial lesions (HSIL), compared to women with normal cytology living in Katowice, Poland. Two sterile swabs were used to obtain material from the posterior vaginal fornix of 143 women with squamous intraepithelial lesions and 39 healthy women: first for general bacteriology, second for detection of urogenital mycoplasmas using Mycoplasma IST2 kit. From each positive Mycoplasma IST2 culture DNA was isolated and PCR was performed for identification of U. parvum and U. urealyticum. Mycoplasma IST was positive in 34.1% cases. Urogenital mycoplasmas were demonstrated in women with HSIL significantly more often compared to women with LSIL, ASCUS, and with normal cytology. DNA of U. parvum was demonstrated in majority of Mycoplasma IST2-positive cases, U. urealyticum DNA-only in 9 (4.9%). Predominance of 3/14 serovars of U. parvum was demonstrated. U. urealyticum biovar 2 was present more often in women with squamous intraepithelial lesions.
Frequent colonization of female and male genitourinary tract by ureaplasmas sometimes hinders evaluation of these microorganisms as infectious agents. Although Ureaplasma urealyticum was divided into two biovars and new species Ureaplasma parvum was described, additional studies are required to determine differences in pathogenicity and respective role of these microorganisms in diseases. Detection of ureaplasmas is possible by characteristic growth on appropriate media and urease activity, but species identification of U. urealyticum and U. parvum must be demonstrated by molecular methods. Differentiation between U. parvum and U. urealyticum is very important, especially for correct interpretation of laboratory results and evaluation of pathogenicity.
U. parvum includes small genome strains: 0.75-0.76 Mpb (former biovar 1 of U. urealyticum-serovars 1, 3, 6, and 14). U. urealyticum includes serovars 2, 4, 5, and 7-13 (former biovar 2) (1-4). Cervical dysplasia is connected with infection with high-oncogenic types of human papillomavirus (HPV). Disruption in function of immune mechanisms and inflammation might be initiated by co-occurrence of bacterial pathogens, including ureaplasmas. The influence of ureaplasmas on cytokine concentrations was confirmed with cell cultures in vitro and in vivo, using animal models and human cervico-vaginal samples (5, 6). Ureaplasmas can also act on progression of cervical dysplasia, classified with the Bethesda System 2001 classification scheme as atypical squamous cells of undetermined significance (ASCUS), low grade squamous intraepithelial lesions (LSIL), high grade squamous intraepithelial lesions (HSIL) and cancer (7).
The aim of this study was to evaluate the occurrence of genital mycoplasmas, especially U. parvum and U. urealyticum, in women with ASCUS, LSIL and HSIL, compared to women with normal cytology in Katowice, southern Poland.
One hundred and eighty two non-pregnant, menstruating, sexually active women (mean age 39.5 yr), with similar socio-economic status, who attended Department of Medical Microbiology of Medical University of Silesia in Katowice for microbiological diagnosis, were included in this study. They did not use oral or vaginal contraceptives and antibiotics/antimycotics within at least 4 weeks before examination. Cervical swabs were taken from all studied women and tested for cytology. All cytology results were interpreted by two pathologists into normal, ASCUS, LSIL, HSIL, squamous cell carcinoma (SCC) according to 2001 Betheda System (7). All women gave informed consent for this study. This study was approved by Bioethical Committee of Medical University of Silesia NN-6501-246/04.
Two sterile swabs were used to obtain material from the posterior vaginal fornix: first for general bacteriology was cultured on the following agar plates: Columbia blood, MacConkey, Chapman, Thayer-Martin and Sabouraud, respectively for streptococci, lactobacilli, Gram-negative rods, staphylococci, gonococci and yeasts. BV-bacterial vaginosis was diagnosed by Nugent and Amsel criteria (8, 9).
Isolated microorganisms were identified with morphological, biochemical and serological characteristics. Second swab for detection of urogenital mycoplasmas was cultured using Mycoplasma IST2 (bioMérieux, Marcy-L'etoile, France), according to manufacturer's instructions. For isolation of DNA, each culture in logarithmic phase of growth was centrifuged 15.000 g for 30 min in 4℃, pellet was twice washed with PBS and incubated with buffer containing proteinase K. Isolation of DNA was performed with appropriate columns, using DNeasy Tissue Kit, Qiagen. Cultures of mycoplasmas in urea-arginine broth and DNAs were stored at -70℃ until used. Two cervical swabs were taken from each of 182 studied women for the determination of Chlamydia trachomatis with AMPLICOR C. trachomatis, Roche Molecular Systems, U.S.A., and high-risk HPV types: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68 by Amplicor HPV, Roche Molecular System U.S.A., respectively.
PCR identification of ureaplasmas was done according to Kong et al. (3), using termocycler Genius (Techne, U.K.) and Taq PCR Master Mix Kit (Qiagen). Primers UMS-57/UMA222 and UMS-170/UMA263 were used for identification of U. parvum (326 bp) and U. urealyticum (476 bp), respectively. Amplified products were visualized under UV light after electrophoresis in 2% agarose gel, containing ethidium bromide (Table 1). The reference strains from American Type Culture Collection: U. urealyticum ATCC 27618 and U. parvum ATCC 27815T were used as positive controls.
Statistical analysis was performed by chi-square test (P<0.05 was statistically significant).
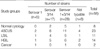
According to cervix cytology results, the following subgroups were identified: 67 women with ASCUS, mean age 40.5 yr; 49 women with LSIL, mean age 36.6 yr; 22 with HSIL, mean age 34.1 yr; and 39 with normal cytology, mean age 39 yr; SCC was diagnosed in 5 cases. Presence of urogenital mycoplasmas was confirmed by Mycoplasma IST2 in 62/182 cases 34.1%. Women with HSIL showed the presence of urogenital mycoplasmas significantly more often than women with LSIL (P<0.001), ASCUS (P=0.005) and normal cytology (P<0.001). In 58 out of 62 Mycoplasma IST2-positive cases DNA of U. parvum was demonstrated while in 9 cases (4.9%) U. urealyticum was detected. In 5 cases both DNAs-U. parvum and U. urealyticum were detected (Table 2). We detected fewer instances of U. urealyticum compared to U. parvum, however, in all but one cases, U. urealyticum biovar 2 strains were isolated from birth canal of women with squamous intraepithelial lesions (Table 2). Among isolated strains of U. parvum predominance of 3/14 serovar was demonstrated (Table 3).
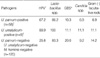
Urogenital mycoplasmas were detected significantly more often in HPV-positive women, compared to HPV-negative women, 57.5% and 18.3%, respectively. All HSIL and cancer cases were HPV-positive. Co-occurrence of selected vaginal microorganisms, including HPV and urogenital mycoplasmas, is presented in Table 4. All women included in this study were C. trachomatis, Neisseria gonorrhoeae and BV-negative.
Commercial mycoplasmal kits showed good correlation with PCR results in detection of Ureaplasma strains, although it is impossible to differentiate U. parvum and U. urealyticum using these commercial kits (10, 11). On top of that, ureaarginine broth used in Mycoplasma IST is suitable for isolation of mycoplasmal DNA (12). This is why in the case of PCR detection we only used samples positive in Mycoplasma IST2. Our results concerning colonization of 32% of tested women with Ureaplasma spp. is in accord with other authors, who demonstrated 20-50% colonization (6, 13-18). However, Abele-Horn et al. in 1997 (19) described 70% positive results. Recently published studies from Eastern Poland show 29.8% occurrence of ureaplasmas in non-pregnant women suffering from urogenital diseases (14). In the case of pregnant women in Central Poland this ratio is 26.3% (6). Our finding of M. hominis (7.7%) is in agreement with our previously described observations as well as with other authors (13, 15, 17). Domination of U. parvum (86.6%) among isolates in our study as well as prevalence of serovar 3/14 was in concordance with others (2, 12, 19-22). Co-occurrence of different mycoplasmal DNAs and presence of different serovars of U. parvum in the same sample demonstrated in our study is in accord with other authors (2, 19, 20).
Only limited publications are available about relations of ureaplasmas with ASCUS, LSIL and HSIL. Lukic and coworkers in 2006 (18) described variations in occurrence of Ureaplasma spp. depending of cytological results: in ASCUS -27%, LSIL -35% and HSIL -45%, compared to women with normal cytology, 19%. The authors concluded that ureaplasmas are an important co-factor for HPV. In our study we demonstrated significantly more frequent occurrence of urogenital mycoplasmas in the group of women with HSIL: DNA of HPV was detected in each women in this group. In our previous studies more frequent isolation of urogenital mycoplasmas in the group of women with LSIL infected with HPV, compared with HPV-negative women, was demonstrated (13). In the present paper we demonstrated that infection with high-risk HPV types often accompanied U. urealyticum 88.9%, less frequently U. parvum 67.2%, and rarely it was confirmed in women without mycoplasmal infection 25.8%. Several studies demonstrated higher frequency of isolation of U. parvum compared with U. urealyticum. In our study the same results were obtained, although percentage of isolated U. urealyticum was very low. In the present work occurrence of U. urealyticum (biovar 2) was 4.9%, but was demonstrated in all but one woman with squamous intraepithelial lesions. Similar results of low percentage of U. urealyticum were demonstrated by Japanese authors in preterm birth group 4.8% (22). In many papers U. urealyticum is indicated as the cause of pathology: in the group of preterm birth women and those with pelvic inflammatory diseases (19), men with nongonococcal and nonchlamydial urethritis (23, 24). Newborns infected with U. urealyticum were subject to more frequent and longer therapeutic procedures supporting respiration, needed more frequent surfactant and antibiotic administration (21). However, controversial data are published as to the role of mycoplasmas in pathogenesis of bronchopulmonary dysplasia and chronic lung disease in newborns (14, 20, 25, 26).
Although our study groups were relatively small, significantly higher occurrence of U. urealyticum in women with squamous intraepithelial lesions suggests a possible role of this bacterium in pathology. Further investigation using larger groups of patients are required to demonstrate possible interactions of microorganisms infected female genital tract and their role in progression of cervical dysplasia.
Figures and Tables
Table 2
Occurrence of urogenital mycoplasmas in study groups (number and percentage of positive cases)

*strains of M. hominis always accompanied ureaplasmas; †occurrence of urogenital mycoplasmas in women with HSIL was significantly higher compared with those with LSIL P=0.001, ASCUS P=0.005 and normal cytology P<0.001.
ASCUS, atypical squamous cells of undetermined significance; LSIL, low grade squamous intraepithelial lesions; HSIL, high grade squamous intraepithelial lesions.
ACKNOWLEDGMENT
The authors gratefully acknowledge Dr. Z. Chelmicki for collecting cervical samples and evaluation of Pap smear.
References
1. Robertson JA, Howard LA, Zinner CL, Stemke GW. Comparison of 16S rRNA genes within the T960 and parvo biovars of ureaplasmas isolated from humans. Int J Syst Bacteriol. 1994. 44:836–838.

2. Kong F, James G, Ma Z, Gordon S, Bin W, Gilbert GL. Phylogenetic analysis of Ureaplasma urealyticum-support for the establishment of a new species, Ureaplasma parvum. Int J Syst Bacteriol. 1999. 49:1879–1889.
3. Kong F, Ma Z, James G, Gordon S, Gilbert GL. Species identification and subtyping of Ureaplasma parvum and Ureaplasma urealyticum using PCR-based assays. J Clin Microbiol. 2000. 38:1175–1179.
4. Robertson JA, Stemke GW, Davis JW Jr, Harasawa R, Thirkell D, Kong F, Shepard MC, Ford DK. Proposal of Ureaplasma parvum sp. nov. and emended description of Ureaplasma urealyticum (Shepard et al. 1974) Robertson et al. 2001. Int J Syst Evol Microbiol. 2002. 52:587–597.

5. Manimtim WM, Hasday JD, Hester L, Fairchild KD, Lovchik JC, Viscardi RM. Ureaplasma urealyticum modulates endotoxin-induced cytokine release by human monocytes derived from preterm and term newborns and adults. Infect Immun. 2001. 69:3906–3915.

6. Kalinka J, Krajewski P, Sobala W, Wasiela M, Brzezińska-Błaszczyk E. The association between maternal cervicovaginal proinflammatory cytokines concentrations during pregnancy and subsequent early-onset neonatal infection. J Perinat Med. 2006. 34:371–377.

7. The Bethesda 2001 Workshop. NCI Bethesda System 2001: 2001 Terminology. Available at: http://www.bethesda2001.cancer.gov/terminology.
8. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med. 1983. 74:14–22.
9. Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991. 29:297–301.

10. Rastawicki W, Kalota H, Jagielski M, Gierczyński R. Comparison of polymerase chain reaction assay and Mycoplasma IST 2 test with culture for detection of infections caused by Ureaplasma urealyticum and Mycoplasma hominis. Med Dosw Mikrobiol. 2004. 56:99–108.
11. Cheah FC, Anderson TP, Darlow BA, Murdoch DR. Comparison of the Mycoplasma Duo test with PCR for detection of ureaplasma species in endotracheal aspirates from premature infants. J Clin Microbiol. 2005. 43:509–510.

12. Pitcher D, Sillis M, Robertson JA. Simple method for determining biovar and serovar types of Ureaplasma urealyticum clinical isolates using PCR-single-strand conformation polymorphism analysis. J Clin Microbiol. 2001. 39:1840–1844.

13. Friedek D, Ekiel A, Chelmicki Z, Romanik M. HPV, Chlamydia trachomatis and genital mycoplasmas infections in women with low-grade squamous intraepithelial lesions (LSIL). Ginekol Pol. 2004. 75:457–463.
14. Kafetzis DA, Skevaki CL, Skouteri V, Gavrili S, Peppa K, Kostalos C, Petrochilou V, Michalas S. Maternal genital colonization with Ureaplasma urealyticum promotes preterm delivery: association of the respiratory colonization of premature infants with chronic lung disease and increased mortality. Clin Infect Dis. 2004. 39:1113–1122.

15. Friedek D, Ekiel A, Romanik M, Chelmicki Z, Wiechula B, Wilk I, Józwiak J, Martirosian G. Co-occurrence of urogenital mycoplasmas and group B streptococci with chlamydial cervicitis. Pol J Microbiol. 2005. 54:253–255.
16. González Bosquet E, Gené A, Ferrer I, Borrás M, Lailla JM. Value of endocervical ureaplasma species colonization as a marker of preterm delivery. Gynecol Obstet Invest. 2006. 61:119–123.
17. Zdrodowska-Stefanow B, Kłosowska WM, Ostaszewska-Puchalska I, Bułhak-Kozioł V, Kotowicz B. Ureaplasma urealyticum and Mycoplasma hominis infection in women with urogenital diseases. Adv Med Sci. 2006. 51:250–253.
18. Lukic A, Canzio C, Patella A, Giovagnoli M, Cipriani P, Frega A, Moscarini M. Determination of cervicovaginal microorganisms in women with abnormal cervical cytology: the role of Ureaplasma urealyticum. Anticancer Res. 2006. 26:4843–4849.
19. Abele-Horn M, Wolff C, Dressel P, Pfaff F, Zimmermann A. Association of Ureaplasma urealyticum biovars with clinical outcome for neonates, obstetric patients, and gynecological patients with pelvic inflammatory disease. J Clin Microbiol. 1997. 35:1199–1202.

20. Katz B, Patel P, Duffy L, Schelonka RL, Dimmitt RA, Waites KB. Characterization of ureaplasmas isolated from preterm infants with and without bronchopulmonary dysplasia. J Clin Microbiol. 2005. 43:4852–4854.

21. Biernat-Sudolska M, Rojek-Zakrzewska D, Rzepecka-Weglarz B, Woźniak J, Lauterbach R. Influence of ureaplasma infection on the clinical state of newborns. Przegl Epidemiol. 2006. 60:53–58.
22. Kataoka S, Yamada T, Chou K, Nishida R, Morikawa M, Minami M, Yamada H, Sakuragi N, Minakami H. Association between preterm birth and vaginal colonization by mycoplasmas in early pregnancy. J Clin Microbiol. 2006. 44:51–55.

23. Yoshida T, Deguchi T, Meda S, Kubota Y, Tamaki M, Yokoi S, Yasuda M, Ishiko H. Quantitative detection of Ureaplasma parvum (biovar 1) and Ureaplasma urealyticum (biovar 2) in urine specimens from men with and without urethritis by real-time polymerase chain reaction. Sex Transm Dis. 2007. 34:416–419.

24. Yokoi S, Maeda S, Kubota Y, Tamaki M, Mizutani K, Yasuda M, Ito S, Nakano M, Ehara H, Deguchi T. The role of Mycoplasma genitalium and Ureaplasma urealyticum biovar 2 in postgonococcal urethritis. Clin Infect Dis. 2007. 45:866–871.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download