Abstract
To evaluate the ability of fluorescence in situ hybridization (FISH) in detecting bladder urothelial carcinoma (BUC), FISH and cytology were compared for the evaluation of 308 consecutive urine samples from patients suspected of having BUC. All patients underwent cystoscopy for identification of bladder lesions. The FISH results were compared with the cytology assessment. In all, 122 patients had confirmed BUC. Among them, 68 (55.7%) were FISH-positive, while only 33 (27%) were positive on cytology. According to disease stage (superficial vs. invasive) and grade (low vs. high), the sensitivities of FISH were also significantly higher than those of cytology in all categories. Moreover, in 36 patients who had no visible tumor with flat, erythematous mucosa (suspicious lesion), FISH was more sensitive than cytology for the detection of BUC (83.3% vs. 33.3%, P=0.002). The FISH was negative in 168 (90.3%) of 186 patients with no histological evidence of BUC or negative cystoscopy findings. The sensitivity of FISH for detecting BUC was superior to that of cytology, regardless of tumor stage and grade. FISH is a significant additional and complementary method for detection of BUC in patients who have suspicious lesions on cystoscopy.
At presentation, the majority of bladder urothelial carcinoma (BUC) is a superficial low- grade tumor which can often be treated with transurethral resection (TUR) and, in some cases, additional intravesical chemo- or immuno-therapy. However, the tumor recurrence rate may be as high as 70% to 80%, and 10% to 15% of recurrent BUC may progress to a higher grade and/or stage, requiring radical cystectomy in most cases (1). Consequently, long-term close surveillance of patients with BUC is essential to manage tumor recurrences and prevent invasive disease.
Cystoscopy and cytology have been the standard diagnostic tools for detection and monitoring of BUC. However, cystoscopy is an invasive technique and can miss the flat lesions such as pTis. Cytology has remained the only alternative noninvasive method for detecting primary BUC or recurrent disease. However, it lacks sensitivity for low-stage and low-grade tumors (2, 3). Therefore, more sensitive and noninvasive methods are under evaluation for the diagnosis and follow-up of patients with BUC. Although many morphology-based, biochemical and molecular methods, including the BTA test, BTA stat, NMP 22 and immunocyt have been shown to be more sensitive than cytology, for the detection of BUC, they lack a comparable level of specificity (4-6).
Genetic abnormalities occur during the initial stages of tumor development and are the primary determinants of neoplastic transformation. Fluorescence in situ hybridization (FISH) is a cytogenetic-based technology that is used for the analysis of multiple chromosomes in several cells. FISH utilizes fluorescently labeled DNA probes that hybridize to the centromeres of the chromosomes or unique loci to detect cells with numerical or structural abnormalities indicative of malignancy. Sokolova et al. (7) reported the development of a FISH assay with high sensitivity and specificity for the detection of UC, using four labeled probes, three centromeric probes for chromosomes 3, 7, and 17 and a one locus-specific probe for chromosome 9. The purpose of the present study was to evaluate FISH for the detection of BUC in routine clinical practice, and compare it to the standard cytology results.
Voided urine specimens from 493 patients were obtained for FISH analysis and cytology examination between April 2006 and July 2007. Among these patients, those who did not undergo cystoscopy (n=152), had a suspected lesion of the upper urinary tract on intravenous urography (IVU) or abdominal ultrasonography (n=20), or were lost to follow-up despite definite identification of bladder lesions on cystoscopy (n=13) were excluded from this study. Finally, voided urine specimens from 308 patients (205 males and 103 females; mean age, 59.7±11.8 yr) were studied. All patients provided their informed consent before participating in this study. Of the 308 patients, the 61 that had a history of UC (bladder: 54, upper tract: 7) and the 247 without a history of UC were evaluated for symptoms suspicious of BUC (microscopic/gross hematuria and obstructive/irritative voiding symptoms). All patients underwent cystoscopy for identification of bladder lesions. One hundred and forty-two patients underwent TUR or biopsies of abnormal bladder lesions. The FISH results were compared with the cytological assessment based on the histopathology and cystoscopy findings.
Cytology samples were centrifuged (1,500 rpm for 5 min) and stained using Papanicolaou's technique. Cytopathologists that were blinded to the patient's clinical information and the result of FISH evaluated the urine samples. The cytology was classified as negative, atypical, suggestive of malignancy, or positive. For the calculation of the sensitivity and specificity, the cases that were interpreted as suggestive of malignancy and positive were pooled together.
FISH was conducted using the instructions in the package insert of the UroVysion™ Bladder Cancer Kit (Abott Molecular Inc, Des Plaines, IL, U.S.A.). The volume of urine available for FISH was more than 33 mL. Cells from voided urine were sedimented at 1,000 rpm for 10 min. Fresh Carnoy's fixative (3:1 [v:v] methanol:glacial acetic acid) was added to the cell pellet; the pellet was mixed and resuspended. Fresh fixative was added a second time; the cells were mixed and placed at -20℃ for 30 min. To ensure appropriate cellular density, 3, 10, and 30 µL of the cell suspension were placed into separate wells on slides. The individual wells were examined under phase contrast and the well demonstrating optimal cell concentration was selected for investigation. Hybridization was performed using UroVysion™ Bladder Cancer Kit (Abott Molecular Inc). The UroVysion probe mixture consists of Chromosome Enumeration Probe (CEP) 3 SpectrumRed, CEP 7 SpectrumGreen, CEP 17 SpectrumAqua and Locus Specific Identifier (LSI) 9p21 SpectrumGold. The probes are pre-mixed and pre-denatured in hybridization buffer for ease of use. Unlabeled blocking DNA is also included with the probes to suppress sequences contained within the target loci that are common to other chromosomes. When hybridized and visualized, these probes provide information on chromosome copy number for chromosome ploidy enumeration. Cells recovered from urine pellets were fixed on slides. The DNA was denatured to its single stranded form and subsequently allowed to hybridize with the UroVysion probes. Following hybridization, the unbound probe was removed by a series of washes, and the nuclei were counterstained with DAPI (4, 6 diamidino-2-phenylindole) and viewed using a fluorescent microscope. At least 25 morphologically abnormal cells were scored. Two independent observers blinded to the clinical information evaluated all samples. In case of disagreement, both observers evaluated the sample again at the same time. Slides were assessed by scanning for cytologically atypical nuclei (larger nuclear size, irregular nuclear shape, and patchy DAPI staining) and then determining the number of CEP3, CEP7, CEP17, and locus-specific probe for chromosome 9 (LSI 9p21). The criteria for a FISH-positive result were those according to Zellweger et al. (8). A sample was considered positive for UC if at least one of the following criteria was met: 1) identification of four or more cells with gains in two or more different chromosomes (3, 7 or 17), 2) observation of homozygous deletion of 9p21 in 12 or more cells.
Pathologists performed the histopathology evaluation on the 142 patients who underwent TUR or biopsy. The stage of BUC was determined based on the International Union Against Cancer TNM classification and tumor grading defined as low (grade 1 and grade 2) or high-grade (grade 3).
The sensitivity of FISH and cytology was determined for the patients with pathology-proven UC, stratified for tumor stage and grade. The specificity of FISH and cytology was calculated for the patients with no histological confirmation of UC or negative cystoscopy findings. The difference between the two methods was determined by the McNemar test. A P value <0.05 was considered statistically significant. SPSS 13.0 (SPSS Inc, Chicago, IL, U.S.A.) was used for statistical calculations.
There were 142 patients with abnormal lesions on cystoscopy, and all of them underwent TUR or biopsy. One hundred and sixty-six patients who did not undergo TUR or biopsy were negative on cystoscopy. Patients had lesions with a variety of growth patterns, including papillary (n=79), nodular (n=14), sessile (n=13), and flat, erythematous mucosa without visible tumor (n=36). In all, 122 patients had confirmed BUC based on the pathology reports. The tumors were pTis in six patients, pTa in 55, pT1 in 41, and pT2 in 20; 78 were low-grade and 44 high-grade.
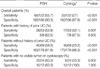
Among the 122 patients with histologically confirmed UC, 30 were FISH-positive with the concurrent cytology results suggestive of malignancy or positive (Fig. 1) and 38 FISH-positive results were discordant (negative or atypical) with the cytology results (Fig. 2). Sixty-eight patients among the 122 with UC were FISH-positive, resulting in an overall sensitivity of 55.7%, while only 33 were positive by cytology (sensitivity: 27%). The difference in the sensitivity between FISH and cytology was statistically significant (Table 1). In addition, the sensitivities of FISH and cytology for the superficial tumors (pTis, pTa, and pT1) were 52% and 25.5%, respectively (P<0.001), while for the muscle-invasive tumors (pT2) they were 75% and 35%, respectively (P=0.021) (Fig. 3). Furthermore, the sensitivities of FISH and cytology were 41% vs. 17.9% for low-grade tumors, and 81.8% vs. 43.2% for high-grade tumors, respectively (P<0.001, P<0.001, respectively) (Fig. 4).
Thirty-six patients who had no visible tumor with flat, erythematous mucosa (suspicious lesions) underwent TUR or biopsies of the bladder lesion. Twenty-four of these patients demonstrated BUC; low grade pTa in 6, high grade pTa in 1, low grade pT1 in 4, high grade pT1 in 4, pTis in 5, and high grade pT2 in 4. Among them, FISH were positive in 20 (83.3%), however, the cytology was positive or suggestive of malignancy in 8 cases (33.3%) (Table 1).
There were 61 patients with a history of UC in this population. Fifty-three of them had documented recurrences and 28 (52.8%) of these patients were FISH-positive; with cytology, 17 (32.1%) were suggestive of malignancy or positive (P=0.007). In the analysis of the 247 patients evaluated for suspicious symptoms without a history of UC, 69 had histologically confirmed UC. The sensitivities of FISH and cytology were 58% and 23.2%, respectively (P<0.001). Among 186 patients with no histological evidence for UC or had negative cystoscopy findings, FISH was negative in 168 (90.3%) and cytology in 182 (97.8%). Although the specificity of cytology was higher than that of FISH, the specificity of FISH overall was 90.3%, and for the patients without a previous UC it was 91.6%.
The use of genetic analysis of urine cells has demonstrated specificity equivalent to that of cytology (9, 10). FISH is used for the detection of cytogenetic abnormalities in malignant cells. Several prior studies (10-12) have reported that FISH was significantly more sensitive than cytology for the detection of UC and that the specificity of FISH and cytology were not significantly different. However, since the confirmation of higher sensitivity of FISH than that of cytology, few investigations for usefulness of FISH have been performed in clinical practice. Moreover, it is unclear which cases did FISH help to detect BUC. Our results also show that FISH had a significantly higher sensitivity than did cytology for the detection of all tumors including superficial/invasive and low/high-grade cases as previously reported. In addition, the sensitivity of FISH was higher than that of cytology for new cases of UC as well as recurrent cases.
The collected volume of urine for FISH or cytology was more than 40 mL, respectively in this study, thus avoiding problems caused by insufficient sample. Nevertheless, the sensitivity of FISH and cytology, in the present study, was unexpectedly lower than in several prior reports (6, 11, 13, 14). The possible explanations for these findings are as follows: 1) Different tumor sizes, grade and stage in each study; it is known that tumor size, grade and stage influence sensitivity and specificity of urine testing for bladder tumor (15, 16). The population of this study included 102 (83.6%) patients with superficial tumors and 78 (63.9%) with low grade tumors. Based on the tumor size, there were 83 patients with tumors 1 cm or less, 23 with 3 cm or less and 16 with tumors more than 3 cm. The tumor characteristics of our population may have resulted in the FISH and cytology sensitivity being lower than that of other studies. 2) The method of collection of the urine specimen, from bladder washing versus voided urine. According to the study of Bhuiyan et al. (17), a comparatively higher sensitivities of urinary tumor marker with cystoscopically collected urine or bladder wash than that of voided urine samples were observed. Urine specimens were exclusively collected from voided urine in this study. 3) The disparity of the study cohorts reflected by the fact that normal healthy volunteers, as well as the patients who underwent transurethral resection of prostate (TURP) for benign prostatic hyperplasia (BPH), were included in other studies as controls (11, 12, 14). All participants in this study were cases under surveillance for BUC in the clinical practice setting. 4) The differences of applied criteria used for FISH positive results among studies. According to several prior reports, a variety of criteria has been applied for FISH positive results, nevertheless, the optimal criteria to define FISH-positive results are not absolutely clear (7, 8, 18, 19). In some investigations, a specimen was considered FISH positive for BC if ten or more cells with gain of a single chromosome or if ten or more cells with homozygous loss of the 9p21 locus (18, 19). In this study, fifteen patients with false-negative FISH had at least one cell with an abnormal signal pattern consistent with polysomy of chromosome 3, 7, 17, and 9p21. We regard our criteria for FISH positive results as more strict than those of previous studies.
Among 59 patients who had had a history of BUC with or without upper tract UC, 19 underwent intravesical immunotherapy. Twelve of these patients were positive on FISH and 9 had a recurred BUC. Because intravesical therapy might cause false-positive result of urine test for detecting BUC (20) and chromosomal integrity was not affected by intravesical therapy (21, 22), it is not likely that intravesical therapy affected FISH result to show lower sensitivity of FISH than other study.
Among 36 patients who had suspicious lesions without visible tumor on cystoscopy, 15 were FISH-positive with discordant results (atypical or negative) on cytology. Thirteen (86.7%) of them had BUC. In the subgroup of 78 patients with low grade superficial tumor (pTa-pT1 with grade 1-2), FISH was positive in 32 (41%), cytology either suggestive of malignancy or positive in 14 (17.9%) and 10 had no definite visible tumor on cystoscopy examination. In these patients, seven patients without visible tumor on cystoscopy were atypical or negative in cytology and 6 of them had the positive results of FISH. However, none of the patients with negative results of FISH had the results suggestive of malignancy or positive in cytology. The FISH-positive result seems to provide a diagnostic clue in detecting BUC for these patients.
FISH missed 54 patients with histologically confirmed UC. The majority of them (51) were negative or atypical on cytology, while only three had results suggestive of malignancy or positive by cytology. Overall, 49 (90.7%) and 46 (85.2%) of 54 false-negative patients by FISH had superficial and low-grade tumors, respectively.
There were 18 false-positive results by FISH. Among them, four patients had results suggestive of malignancy or positive by cytology; TUR or biopsy was performed in two patients with abnormal lesions on cystoscopy. The histopathology of these cases was chronic inflammation and reactive urothelium without malignancy. According to the report of Sarosdy et al. (14), patients with false-positive FISH had a significantly higher recurrence rate than those with a true-negative result. Another investigation reported that multi-target FISH might help to stratify the risk of UC recurrence, at the time of a negative cystoscopy, by using optimal criteria for FISH positive results (8). Although two patients with false-positive FISH had BUC diagnosed during follow-up, it is hard to assess the clinical implication of the false-positive FISH results because of the limited follow-up duration of this study.
The limitations of this study include that it was a retrospective study and that the histopathology results were not available in all patients because TUR or biopsy was determined by cystoscopy finding. However, cystoscopy, considered as the gold standard for the detection of BUC, was performed to identify the presence or absence of bladder lesions in all patients. Moreover, to exclude the influence of upper tract UC on the analysis, the study population was restricted to only patients being evaluated for BUC. Despite these limitations, this study is one of few studies that compared the performance characteristics of FISH and cytology in clinical practice setting, unlike other studies which included normal healthy volunteers and the patients with BPH as controls.
In conclusion, the sensitivity of the FISH assay using chromosomes 3, 7, 17, and 9p21 for the detection of BUC was superior to that of urine cytology, regardless of the tumor stage and grade, although FISH was slightly less specific than cytology. Even if taking its high cost into consideration, FISH may be a significant additional and complementary method for the detection of BUC, especially in patients who have no visible tumor but flat, erythematous mucosa (suspicious lesion) on cystoscopy.
Figures and Tables
Fig. 1
A case with high-grade T1 (T1G3) urothelial carcinoma, cytology suggestive of malignancy, and positive FISH. Cytology, Pap stain 100×showing clusters of pleomorphic nuclei with large nucleoli, suggestive of malignancy (A). FISH shows four copies of chromosome 3 (red), three copies of chromosome 7 (green) and six copies of chromosome 17 (aqua) (B). Resected tumor (H&E, ×20) demonstrates invasion of lamina propria by high grade tumor (C).

Fig. 2
A case with high grade Ta (TaG3) urothelial carcinoma, negative cytology, and positive FISH. Cytology, Pap stain 100×showing normal urothelium, negative cytology (A). FISH shows four copies of chromosome 3 (red) and 7 (green), and three copies of chromosome 17 (aqua) (B). Resected tumor (H&E, ×20) shows papillary high-grade tumor confined to epithelium (C).

Fig. 3
Sensitivity based on histologic stage.
FISH, fluorescence in situ hybridization; Superficial, Tis, Ta, and T1 urothelial carcinoma; Invasive, ≥T2 urothelial carcinoma.

References
1. Raghavan D, Shipley WU, Garnick MB, Russell PJ, Richie JP. Biology and management of bladder cancer. N Engl J Med. 1990. 322:1129–1138.

2. Maier U, Simak R, Neuhold N. The clinical value of urinary cytology: 12 years of experience with 615 patients. J Clin Pathol. 1995. 48:314–317.

3. Konety BR, Getzenberg RH. Urine based markers of urological malignancy. J Urol. 2001. 165:600–611.

4. Lokeshwar VB, Soloway MS. Current bladder tumor tests: does their projected utility fulfill clinical necessity? J Urol. 2001. 165:1067–1077.

5. Glas AS, Roos D, Deutekom M, Zwinderman AH, Bossuyt PM, Kurth KH. Tumor markers in the diagnosis of primary bladder cancer. A systematic review. J Urol. 2003. 169:1975–1982.

6. Lotan Y, Roehrborn CG. Sensitivity and specificity of commonly available bladder tumor markers versus cytology: results of a comprehensive literature review and meta-analyses. Urology. 2003. 61:109–118.

7. Sokolova IA, Halling KC, Jenkins RB, Burkhardt HM, Meyer RG, Seelig SA, King W. The development of a multitarget, multicolor fluorescence in situ hybridization assay for the detection of urothelial carcinoma in urine. J Mol Diagn. 2000. 2:116–123.

8. Zellweger T, Benz G, Cathomas G, Mihatsch MJ, Sulser T, Gasser TC, Bubendorf L. Multi-target fluorescence in situ hybridization in bladder washings for prediction of recurrent bladder cancer. Int J Cancer. 2006. 119:1660–1665.
9. Mao L, Schoenberg MP, Scicchitano M, Erozan YS, Merlo A, Schwab D, Sidransky D. Molecular detection of primary bladder cancer by microsatellite analysis. Science. 1996. 271:659–662.

10. Halling KC, King W, Sokolova IA, Meyer RG, Burkhardt HM, Halling AC, Cheville JC, Sebo TJ, Ramakumar S, Stewart CS, Pankratz S, O'Kane DJ, Seelig SA, Lieber MM, Jenkins RB. A comparison of cytology and fluorescence in situ hybridization for the detection of urothelial carcinoma. J Urol. 2000. 164:1768–1775.

11. Placer J, Espinet B, Salido M, Sole F, Gelabert-Mas A. Clinical utility of a multiprobe FISH assay in voided urine specimens for the detection of bladder cancer and its recurrences, compared with urinary cytology. Eur Urol. 2002. 42:547–552.

12. Marin-Aguilera M, Mengual L, Ribal MJ, Burset M, Arce Y, Ars E, Oliver A, Villavicencio H, Algaba F, Alcaraz A. Utility of a multiprobe fluorescence in situ hybridization assay in the detection of superficial urothelial bladder cancer. Cancer Genet Cytogenet. 2007. 173:131–135.
13. Halling KC, King W, Sokolova IA, Karnes RJ, Meyer RG, Powell EL, Sebo TJ, Cheville JC, Clayton AC, Krajnik KL, Ebert TA, Nelson RE, Burkhardt HM, Ramakumar S, Stewart CS, Pankratz VS, Lieber MM, Blute ML, Zincke H, Seelig SA, Jenkins RB, O'Kane DJ. A comparison of BTA stat, hemoglobin dipstick, telomerase and Vysis UroVysion assays for the detection of urothelial carcinoma in urine. J Urol. 2002. 167:2001–2006.

14. Sarosdy MF, Schellhammer P, Bokinsky G, Kahn P, Chao R, Yore L, Zadra J, Burzon D, Osher G, Bridge JA, Anderson S, Johansson SL, Lieber M, Soloway M, Flom K. Clinical evaluation of a multitarget fluorescent in situ hybridization assay for detection of bladder cancer. J Urol. 2002. 168:1950–1954.

15. Raitanen MP, Marttila T, Kaasinen E, Rintala E, Aine R, Tammela TL. Sensitivity of human complement factor H related protein (BTA stat) test and voided urine cytology in the diagnosis of bladder cancer. J Urol. 2000. 163:1689–1692.

16. Boman H, Hedelin H, Holmang S. Four bladder tumor markers have a disappointingly low sensitivity for small size and low grade recurrence. J Urol. 2002. 167:80–83.

17. Bhuiyan J, Akhter J, O'Kane DJ. Performance characteristics of multiple urinary tumor markers and sample collection techniques in the detection of transitional cell carcinoma of the bladder. Clin Chim Acta. 2003. 331:69–77.

18. Bubendorf L, Grilli B, Sauter G, Mihatsch MJ, Gasser TC, Dalquen P. Multiprobe FISH for enhanced detection of bladder cancer in voided urine specimens and bladder washings. Am J Clin Pathol. 2001. 116:79–86.

19. Junker K, Fritsch T, Hartmann A, Schulze W, Schubert J. Multicolor fluorescence in situ hybridization (M-FISH) on cells from urine for the detection of bladder cancer. Cytogenet Genome Res. 2006. 114:279–283.

20. Raitanen MP, Kaasinen E, Lukkarinen O, Kauppinen R, Viitanen J, Liukkonen T, Tammela TL. Analysis of false-positive BTA STAT test results in patients followed up for bladder cancer. Urology. 2001. 57:680–684.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download