INTRODUCTION
Neonatal respiratory distress syndrome (RDS) is a progressive respiratory failure that is caused primarily by a deficiency of pulmonary surfactants (PS). In 1959, Avery and Mead (
1) showed that PS deficiency is a major factor in the pathophysiology of RDS. The first successful treatment was reported in 1980, when Fujiwara et al. (
2) successfully administered exogenous PS to preterm infants with RDS. PS replacement therapy is now the routine method of treatment for infants with RDS, and since its introduction, morbidity and mortality due to RDS in preterm infants has decreased remarkably.
In Korea, Surfacten® (Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan), the first artificial PS, was available in 1991. Thereafter, Exosurf® (Burroughs Wellcome Co., Research Triangle Park, NC, U.S.A.) was available from 1991 to 1997, Newfactan® (Yuhan Pharm Corporation, Seoul, Korea) from 1996, and Curosurf® (Chiesi Farmaceutici, Parma, Italy) from 2003 (
Table 1).
In Korea, PS was first used to treat 8 RDS cases in 1990 by Namgung et al. (
3), and this was followed by a report on 6 cases by Park et al. (
4) in 1991. Subsequently, Bae et al. (
5) reported PS therapy outcomes in neonatal RDS on a national basis for 1990 and 1991. Bae et al. published again a report on the clinical outcomes of PS therapy in Korea in 1996 and 2002, respectively (
6,
7).
In the present study, we investigated improvements of clinical outcome achieved by PS therapy for the treatment of RDS on national data basis for the year of 2007 and compared these findings with those of the three previous reports.
DISCUSSION
In 1980, Fujiwara et al. (
2) first successfully administered exogenous PS to preterm infants with RDS, and subsequently several PS preparations were developed. These preparations have since been shown to reduce mortality and morbidity effectively among RDS patients by several double blind, randomized, controlled studies. Today PS replacement therapy has become a routine method of treating infants with RDS.
The PS preparations used today are either animal-sourced or synthesized. Animal-sourced agents, include; 1) Alveofact® (Bovactant, Germany), BLES® (Canada), Infasurf® (Calfactant, U.S.A.), Survanta® (Beractant, U.S.A.), Surfacten® (Surfactant-TA, Japan), and Newfactan® (Korea), which are all extracted from bovine lungs, and 2) Curosurf® (Poractant alfa, Italy) from porcine lungs. Synthetic agents include; ALEC® (Pulmactant, U.K., no longer manufactured), Exosurf® (Colfosceril palmitate, U.S.A.); phospholipid synthetics, and Surfaxin® (Lucinactant, U.S.A., not FDA licensed), a surfactant protein B synthetic (
11). Of these, Surfacten®, Newfactan®, and Curosurf® are currently being used, but Exosurf®, which was used for short time, is no longer available in Korea. The PS agents used in Korea are listed in
Table 1.
Surfacten® and Newfactan® contain phospholipids extracted from bovine lung, and surfactant proteins -B and -C, which increase surface physical activity and reduce surface tension on the alveolar surface. Curosurf® is produced from porcine lung by chloroform/methanol extraction and liquid gel electrophoresis for purification, and contains surfactant proteins -B and -C. In Group IV, Surfacten® (51.3%) was administered in more than half cases, and Newfactan® (39.3%), Curosurf® (8.3%), and mixtures of these agents (1.1%) were followed. Several meta-analyses concluded that PS replacement therapy reduces mortality in RDS patients. In 1993, Jobe et al. (
12) after integrating results from 1985 to 1992, reported that mortality is decreased by PS therapy when used as a prophylactic or rescue therapy with an odds ratio of 0.6. Furthermore, in 1993 Ptamanik et al. (
13) reported that PS agents of synthetic or animal origin administered as prophylactic or rescue therapies effectively reduce mortality among RDS patients (odds ratio 0.5-0.6). In a meta-analysis conducted by the Committee on Fetus and Newborn of the American Academy of Pediatrics (2008) (
14), attributed remarkable reductions in the mortalities of RDS patients to these agents (odds ratio of 0.6 for agents of animal origin and of 0.7 for those of synthetic origin when administered with prophylactic intent; and of 0.67 for agents of animal origin PS and 0.73 for those of synthetic origin when administered as rescue therapies).
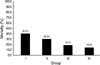
The use of PS therapy obviously decreased mortality among Korean RDS patients over the 17-yr study period, i.e., from 40.0%, 30.0%, 18.7%, and 14.3% for the periods 1990/91, 1996, 2002, and 2007, respectively. Furthermore, PS therapy reduced mortalities not only among RDS patients, but also among prematurities and neonates in Korea. In the pre-PS era (before 1990), mortality due to RDS was much higher. In addition, several advances in neonatal supportive care, such as, improvements in artificial ventilator strategies, and the prevention and early management of infections and complications, have also undoubtedly contributed to these reductions in mortality (
15-
17).
A double blind, randomized, controlled study on Surfacten® conducted in Japan found lower mortality among RDS patients (8%, birth weight<1,750 g) than we found in the present study (
9). On the other hand, other double blind, randomized, controlled study performed on Survanta®, which has the same composition as Surfacten®, reported a RDS mortality of 18.4% (birth weight <1,750 g) (
18).
In the present study, we investigated the frequency of RDS among in-hospital-born patients in 2007 treated at 57 hospitals and found that the frequency of RDS decreases as birth weight is light or gestational period is short.
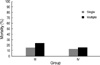
Furthermore, in the present study, PS was administered to a greater proportion of RDS patients with time, i.e., 47.3%, 66.2%, and 77.7% in Groups II, III, and IV, respectively. This appears to be due to changed attitude of the Korean Medical Insurance Association in terms of PS administration. In terms of our 2007 data, rates of PS use were found to be greater in patients with a smaller gestational period, which implies that RDS is more severe among these patients. Furthermore, PS replacement therapy was used in 87.9% of low birth weight infants (<2,500 g) and in 56.7% of very low birth weight infants (<1,500 g) in terms of birth weight, and in 93.5% of preterm infants in terms of gestational period (especially 73% had a gestational period of <32 weeks). In brief, infants with a gestational period of <32 weeks and a birth weight of <1,500 g were major members.
In terms of the classification of initial chest radiographic findings in RDS patients, stage distributions were similar for Groups III and IV. The proportions of patients with stage III or IV (severely affected) composed about 2/3 of Groups III and IV, which confirms that patients with more severe initial chest findings by radiography require more PS therapy.
Initial respiratory distress classifications using ventilatory index showed that patients who received PS therapy in Groups I and II had milder conditions than those in Groups III and IV, which appears to be due to the extended indication for PS referred to above. Groups III and IV had similar initial respiratory distress distributions. In Group IV, 23.0% had mild respiratory distress, 42.0% had moderate, and 35.0% had severe respiratory distress. Furthermore, those with moderate or severe respiratory distress constituted as much as 77% of Group IV RDS patients, and thus, more aggressive management is required in these patients.
Korean medical insurance policies did not permit multiple administration of PS in 1990/91 (Group I) or in 1996 (Group II), when only a single dose was administered in all cases. However, the policy was changed to a direction permitting multiple dose in 2001. In Groups III and IV multiple administrations were performed in roughly 1/4 of cases. In Groups III and IV 14.4% and 16.0% of patients relapsed and these patients were treated with multiple doses of PS.
The classification of early response to the PS treatment showed better results in Groups III and IV than in Groups I and II. In Group IV, 69.2% responded well, 14.4% relapsed, and 16.4% responded poorly, which means that about 1/5th of RDS patients relapsed after initial PS treatment. In a Japanese report (
10), 79% responded well, 16% relapsed, and 5% responded poorly, which is a substantially better response rate that observed in our 2007 study.
To improve the prognosis of RDS patients early detection and adequate management of co-morbidities, including associated diseases and complications, are as important as general supportive care. We examined data for the past 17 yr in terms of complications and associated diseases that developed during the management of RDS patients and preterm babies (
Table 5).
Mortalities of RDS neonates in Korea were found to dramatically improve over the 17-yr study period, i.e., from 40.0%, 30.0%, 18.7%, to 14.3% for 1990/91, 1996, 2002, and 2007, respectively. Furthermore, our study of birth weights and gestational periods showed that both are inversely related to mortality, which means that these high-risk patients require more attention.
In this longitudinal study, we analyzed clinical findings, disease severity, dose schedule of PS administration, response to treatment, associated diseases, complications, and mortalities, with respect to RDS and PS treatment in neonates over a 17-yr period in Korea. We conclude that surfactant treatments in neonates with RDS have had a marked impact on clinical course and outcome, together with substantial reduction of mortality rates among RDS patients over the last 17 yr in Korea.
LIST OF CONTRIBUTORS
We list the following 57 hospitals and individuals who participated in this research by providing information in this nationwide study; Byeong Il Kim (Seoul National University Bundang Hospital), Chang Ryul Kim (Hanyang University Guri Hospital), Chan Hoo Park (Gyeongsang National University Hospital), Chong Woo Bae (Kyunghee University East-West Neo Medical Center), Chung Sik Chun (The Catholic University of Korea Kangnam St. Mary's Hospital), Eun Ae Park (Ewha Womans University Mokdong Hospital), Eun Sil Lee (Yeungnam University Medical Center), Han Suk Kim (Seoul National University Children's Hospital), Heng Mi Kim (Kyungpook National University Hospital), Hye Sun Yoon (Eulji General Hospital), Hyun Kyung Park (Hanyang University Medical Center), Hyun Seung Jin (Gangneung Asan Hospital), Hyun Seung Lee (The Catholic University of Korea Uijeongbu St. Mary's Hospital), Il Sung Park (Soonchunhyang University Gumi Hospital), Il Tae Hwang (Hallym University Kangdong Sacred Heart Hospital), In Kyung Sung (The Catholic University of Korea St. Mary's Hospital), Jang Hoon Lee (Korea University Anam Hospital), Jang Hoon Lee (Korea University Ansan Hospital), Jong Hee Hwang (Inje University Ilsan Paik Hospital), Jung Are Kim (Hanil General Hospital), Jung Hyun Lee (The Catholic University of Korea St. Vincent's Hospital), Jung Ju Lee (Chung-Ang University Medical Center), Ki Soo Kim (University of Ulsan Asan Medical Center), Kyu Hyung Lee (Pochon Jungmun University Cha Hospital), Kyung Hee Lee (Gumi CHA General Hospital), Kyung Og Ko (Konyang University Hospital), Kyun Woo Lee (Dae Dong Hospital), Mea Young Chang (Chungnam National University Hospital), Mi Jung Kim (Chungbuk University Hospital), Mi Lim Koo (Sowha Children's Hospital), Min Soo Park (Yonsei University Yongdong Severance Hospital), Moon Sung Park (Ajou University Hospital), Myoung Jae Chey (Inje University Sanggye Paik Hospital), Myung Ho Oh (Soonchunhyang University Cheonan Hospital), Ran Namgung (Yonsei University Severance Children's Hospital), Sang Geel Lee (Daegu Fatima Hospital), Sang Kee Park (Chosun University Hospital), Sang Lak Lee (Keimyung University Dongsan Medical Center), Sang Young Bae (Halla General Hospital), Son Moon Shin (Cheil General Hospital & Women's Health Care Center), Soo Chul Cho (Chonbuk National University Hospital), So Young Kim (The Catholic University of Korea Holy Family Hospital), Su Eun Park (Pusan National University Hospital), Sung Mi Kim (Busan St. Mary's Medical Center), Sung Min Choi (Dongguk University Gyongju Hospital), Sung Shin Kim (Soonchunhyang University Bucheon Hospital), Sun Ju Lee (Dongguk University Pohang Hospital), Tae Jung Sung (Hallym University Kangnam Sacred Heart Hospital), Won Ho Hahn (Kyunghee University Kyunghee Medical Center), Woo Taek Kim (Daegu Catholic University Medical Center), Yeun Kyun Oh (Wonkwang University Hospital), Yong Hoon Jun (Inha University Hospital), Yong Won Park (College of Medicine Inje University Seoul Paik Hospital), Young Hee Kim (Bundang Jaesang General Hospital), Young Sook Hong (Korea University Guro Hospital), Young Youn Choi (Chonnam National University Hospital), and Youn Woo Kim (Cheju National University Hospital).















 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download