Abstract
Previously published studies on Kikuchi disease (KD) have frequently addressed the computed tomography (CT) findings in the adult population, however, only a few studies have been reported for the pediatric age group. The purpose of this study is to analyze the clinical characteristics and imaging features of KD in children. Fifteen children (2-14 yr) who had a neck CT and pathology diagnosis of KD were included in this study. Clinical features, including the duration of lymphadenopathy and fever, prognosis, and laboratory values, were evaluated. We analyzed the sites, size, and lymph node pattern as seen on their CT scans. The median duration of fever was 10 days. Fourteen patients experienced improvement in their condition, although four of these patients experienced recurrent episodes of KD. All patients had affected cervical nodes at level V. Perinodal infiltrates were observed in the affected cervical nodes in 14 cases (93%), and non-enhancing necrosis was also noted within the affected cervical nodes in 10 cases (63%). In conclusion, the combination of imaging findings in conjunction with clinical findings of KD may help to determine whether or not to perform pathology analysis and follow-up studies.
Kikuchi disease (KD) or histiocytic necrotizing lymphadenitis is a self-limiting disorder of unknown origin; KD is characterized by painful cervical lymphadenopathy with prolonged fever (1). Therefore, although rare, KD should be part of the differential diagnosis of posterior cervical lymphadenopathy in children, especially in Asian pediatric patients (1, 2).
As most KD patients recover spontaneously and are subsequently followed in outpatient clinics, accurate diagnosis can avoid both unnecessary procedures and treatment. Computed tomography (CT) findings as well as the clinical characteristics of KD have already been reported, however, only a few published studies have analyzed the characteristics of KD in pediatric patients. Therefore, the authors retrospectively reviewed the clinical manifestations and imaging features of KD in a pediatric cohort.
Between March 2001 and December 2007, a total of 15 children with a pathologic diagnosis of KD and who underwent neck CT, were included in this study. Specimens from the excisional biopsies of the cervical lymph nodes at level V showed necrotizing lymphadenitis and confirmed the diagnosis of KD in all patients. Indications for biopsy included lymph nodes in the supraclavicular region of the neck, prolonged fever more than 7 days and increase in the size of nodes which failed to respond to antibiotic therapy.
We retrospectively analyzed both the medical records and the imaging findings of these patients. The medical records of all 15 patients were retrospectively reviewed in order to identify the clinical and laboratory features including the age/gender, duration of fever before admission, leukocyte count, hemoglobin level, platelet count, lactate dehydrogenase (LDH), the erythrocyte segmentation rate (ESR), the C-reactive protein (CRP), treatment response, and associated diseases (Table 1).
A total of 15 neck CT scans, 13 neck ultrasound scans (US), three abdominal US, three abdominal CT, and two 2-[fluorine 18]-fluoro-2-deoxy-D-glucose (FDG)-Positron Emission Tomography (PET) scans were performed on these fifteen children.
CT examinations (Somatom Plus, Siemens Medical Systems, Erlangen, Germany) were performed following intravenous administration of non-ionic contrast medium (Ultravist: Schering, Berlin, Germany). Neck CT was performed using axial scans from the skull base to the upper mediastinum. The ultrasound exam was performed on an ATL HDI Ultramark 9 scanner (Bothell, WA, U.S.A.). We analyzed the sites, size (greatest dimension), shape, perinodal infiltration, and contrast enhancement pattern of the affected lymph nodes (Table 2). The sites of lymph nodes identified by imaging were analyzed using Som's classification (3). The diagnostic CT criteria for abnormal lymphadenopathy included 1) increased size (compared with the expected normal size of the respective region), 2) lymph nodes of any size with central necrosis, perinodal infiltrates, and increased contrast enhancement, and 3) nodal grouping and the presence of three or more contiguous and confluent lymph nodes. On CT scanning, perinodal infiltration was visualized as obliteration or increased attenuation of the adjacent fat planes.
US and FDG-PET scans were also analyzed in patients with available data. On gray-scale US images, we analyzed the presence of the echogenic nodal hilum or internal anechoic area and the sharpness of the border. We did not analyze color Doppler images as they were only obtained in some of our study patients. The FDG-PET data were evaluated qualitatively for regions of focally increased glucose metabolism. This study was reviewed and approved by our hospital's human rights committee.
Our clinical and laboratory findings are detailed in Table 1. There were 11 boys and 4 girls with a mean age of 10.5 yr (age range: 2-14 yr) and a male-female ratio of 2.8:1. The median duration of fever before admission was 10 days (6-60 days). Laboratory findings included leukopenia (80%), leukocytosis (13%), anemia (67%), and elevated LDH (93%). ESR and CRP levels were elevated in all 15 patients. Five patients had KD associated hemophagocytic lymphohistiocytosis (HLH). Fourteen improved within five months (8 weeks-5 months), although four patients had recurrent episodes of KD occurred 11-26 months after their initial episodes. The follow-up period averaged 3.2 yr.
Ten patients recovered without specific therapy after excisional biopsy, and five patients were treated with prednisolone. All patients treated with a steroid had both KD and HLH. In three of the five patients with both KD and HLH who failed to respond to corticosteroid treatment, chemotherapy, i.e. etoposide, dexamethasone or cyclosporine-A, was started. The condition of other two children improved. One child treated with chemotherapy died from secondary disseminated coagulopathy caused by a buccal abscess.
The CT findings are summarized in Table 2. All patients had affected cervical nodes at level V. Other frequently affected nodes were at levels IV (n=14), II (n=13), III (n=13), and I (n=6); the supraclavicular nodes (n=6) were also affected. Two cases were also noted to have parotid (n=1) and retroauricular (n=1) nodes. Six patients (40%) had bilateral neck involvement. Nine (60%) patients presented with unilateral nodal involvement, mostly occurring on the left side (7/9).
In our series, all affected lymph nodes appeared as clusters of small- to medium-sized (1.8 to 3.1 cm, mean: 2.3 cm) nodes with oval to round shape (Figs. 1-3). Perinodal infiltrates were seen in the affected cervical nodes in 14 (93%) of our patients (Fig. 1-3). On enhanced CT, non-enhancing necrosis within the affected cervical nodes was also noted in ten patients (63%) (Fig. 1).
In five patients (33%), all affected nodes showed uniform enhancement without necrosis (Fig. 2). Although the lymph node attenuation was less intense than that of the vascular structure, it was higher than that of the adjacent musculature (Fig. 2).
Abdominal imaging was only performed in three patients (Fig. 3), all of whom demonstrated extensive lymphadenopathy involving many nodal groups in the inguinal (n=2), paraaortic (n=2), iliac (n=1), peripancreatic (n=1), and obturator (n=1) regions. Whole-body FDG PET was performed on two of these three patients and revealed multiple nodes demonstrating FDG-avid nodular masses in right cervical and supraclavicular nodes (standardized uptake value, SUV, 10.4) and abdominal nodes (SUV, 10) (Fig. 3C). Features of the perinodal infiltrates were well-visualized on both CT and US, but regions of internal necrosis were not visualized on either modality (Fig. 3). The imaging features in our patients with KD associated with HLH did not differ from those in the patients with KD only.
KD is characterized by fever and self-limiting tender cervical lymphadenopathy as well as by the histological characteristics of histiocytic necrotizing lymphadenitis (1, 2). Although rare, KD should be included in the differential diagnosis of posterior cervical lymphadenopathy in children, especially in Asian pediatric patients (1, 2).
Regarding the age at onset, a previous study with an adult population found that KD has a female predilection with a 3-4:1 ratio (4). However, the present study which was performed in a pediatric population, showed a completely different gender predominance, i.e. KD was found to be more prevalent in boys than in girls (Male/Female, 2.8:1). A previous study with a pediatric population reported a similar gender predilection, i.e. M/F of 1.9:1 (2). Therefore, it is unclear why KD predominantly affects women in adult populations, but boys in pediatric populations younger than 16 yr of age. In the present study, the youngest child was two years old, and three patients (20%) were less than 10 yr of age.
Laboratory tests are helpful in ruling out causes of cervical lymphadenopathy, but no specific tests are available for detecting KD. KD patients may have mild leukopenia, elevated ESR and CRP or rarely, leukocytosis. Leukopenia has been reported to occur in 16.6% to 58.3% of KD patients and was observed in 80% of our 15 pediatric patients. In a previous study of pediatric KD patients, elevated ESR and CRP were noted in 72.7% and 72.2%, respectively, but in our study, all patients demonstrated elevated ESR and CRP levels (2). The LDH level in our series was generally increased (93%). Moreover, as found in adults in other studies, the laboratory indices of almost all of our study patients examined during the febrile period, showed leukopenia, anemia, and elevated ESR and LDH values.
In the present study, the imaging features of KD did not differ markedly from those in some general series (5, 6). Cervical lymph nodes in KD have a tendency to be located in the posterior triangle nodes, as seen in 48% to 77% of the cases in previous literature reports (5, 6), however, this tendency was more obvious in the present study in which all patients had affected nodes at level V. Moreover, in our study, all affected lymph nodes appeared as clusters of small- to medium-sized lymph nodes and no nodes exceeded 3 cm in greatest dimension. Therefore, the nodes appeared abnormal not because of their sizes but rather because of their numbers and enhancement features.
Cervical nodes are most commonly affected by KD, but virtually any nodal chain can be involved in KD and axillary, supraclavicular, mediastinal, peripancreatic, iliac, and inguinal locations of nodes have been reported, as was observed in three of our study cases (6, 7). Isolated intra-abdominal adenopathy has only rarely been reported, however, as imaging of the thorax and abdomen is not usually performed, it is possible that to date thoracic and abdominal disease involvement has not been detected (7).
US is generally used as the modality of choice for evaluating cervical lymphadenopathy in children because it has no radiation hazard. In the present study, US was also performed but was found to be of limited use in terms of evaluating internal necrosis of affected nodes. Unfortunately, only a limited number of our study patients were evaluated on color Doppler US and this number was too small to allow us to analyze the significance of the imaging findings. In all four patients who underwent color Doppler US, it showed hilar vascularity, however, CT demonstrated eccentric necrosis in these cases. A previous report of the Korean population mentioned that color Doppler US findings of KD include symmetrical central hilar vascularity with some flow signals in the peripheral soft tissue which are similar to those of benign lymphadenitis (8). Further studies of the utility of color Doppler US in diagnosing KD, are needed in the pediatric population.
Although CT poses a radiation hazard, it is the preferred modality for evaluating perinodal infiltrates (93%) and nodal necrosis (63%), both of which were frequently observed in our patients. The cause of the more frequent nodal necrosis in this pediatric series than in previous adult series, is unclear. However, the difference may be attributable to the fact that the clinical course and immune response of KD differ according to individual patients and their ages (1). Another factor contributing to this difference is thought to be stage of the histopathologic changes in KD associated with its clinical course. Although the early proliferative stage of KD with its lymphohistiocytic proliferation and numerous atypical mononuclear cells, can cause it to be confused with malignant lymphoma, cortical and paracortical necrosis are the chief histopathologic features of KD (1, 7), and some previous literature reports have indicated the rare occurrence of extensive necrosis (7). However, in our series, full-blown macroscopic necrosis of affected nodes was not unusual on CT, especially in febrile children. Clinically and radiologically, KD could lead to a misdiagnosis of tuberculosis or even malignant lymphoma (5, 7). In one of our patients, multiple homogenously enhancing nodes without nodal necrosis were seen on CT, and multiple FDG-avid nodular masses in both cervical and abdominal lymph nodes was seen on FDG PET scans, thus mimicking lymphoma. Previous PET studies have indicated that affected nodes in KD demonstrate FDG uptake (standardized uptake value, SUV) compatible with malignant adenopathy (9). Although qualitative and quantitative analysis of regions of focally increased glucose metabolism seen on PET may falsely indicate malignant lymphoma, a finding of enlarged nodes <3 cm in greatest dimension and with perinodal infiltrates, might be helpful in differentiating pediatric KD from lymphoma.
Most previous reports have suggested that nodes affected be KD resolve spontaneously without specific treatment within six months of biopsy; this was also observed in most of our pediatric patients (1, 2). The rate of KD recurrence has been reported to range from 1.3-4%, however, 27% (n=4) of our 15 patients experienced recurrent episodes at least one year after the initial resolution (2, 4). Furthermore, two of four of these patients had recurrent KD-associated HLH.
In conclusion, the present study demonstrates that KD in children has somewhat different clinical manifestations and imaging features compared with previously reported results in adult populations. Our study demonstrates that KD shows a male predominance in children which contrasts to the female predominance among young adults. Furthermore, in the present study, KD was found to have higher rates of recurrence and HLH development than have been previously reported.
In our pediatric series, the most common CT feature of KD was clusters of multiple, small- to medium-sized ovoid to round lymph nodes with internal necrosis and perinodal infiltrates at level V. The authors advise that awareness of the CT features and clinical characteristics of KD in children may help to determine whether or not to perform pathology analysis and follow- up studies.
Figures and Tables
Fig. 1
A 12-yr-old boy with fever and cervical lymphadenopathy. Axial CT scans of the neck show multiple, small- and medium-sized lymph nodes in both submandibular regions (levels IA) (arrows) and on the right side of the neck (level V) (arrowheads). Note the ring-shaped nodular necrosis (*) on the right side of the neck as A well as the adjacent perinodal infiltration.
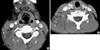
Fig. 2
A two-year-old girl with fever and bilateral cervical lymphadenopathy. Axial CT scans show multiple, medium-dimension lymph nodes with uniform enhancement on both sides of the neck at levels II and V. Note the increased attenuation in the adjacent superficial space (arrows).
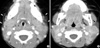
Fig. 3
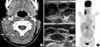
A 10-yr-old girl with intermittent fever and right cervical lymphadenopathy. (A) CT scan shows multiple, small- and medium-sized lymph nodes on the right side of the neck (arrows) (levels IIB and V). Note the perinodal fat obliteration in the adjacent superficial space (arrowhead). (B) US obtained at the same time show somewhat matted, hypoechoic, nodal masses with irregular perinodal borders, suggestive of perinodal infiltrates. (C) MIP FDG-PET scan image shows multiple areas of FDG uptake in the right cervical, supraclavicular, right para-aortic and peripancreatic regions.
References
1. Lee KY, Yeon YH, Lee BC. Kikuchi-Fujimoto disease with prolonged fever in children. Pediatrics. 2004. 114:e752–e756.

2. Lin HC, Su CY, Huang SC. Kikuchi's disease in Asian children. Pediatrics. 2005. 115:e92–e96.
3. Som PM, Curtin HD, Mancuso AA. Imaging-based nodal classification for evaluation of neck metastatic adenopathy. AJR Am J Roentgenol. 2000. 174:837–844.

4. Tsang WY, Chan JK, Ng CS. Kikuchi's lymphadenitis. A morphologic analysis of 75 cases with special reference to unusual features. Am J Surg Pathol. 1994. 18:219–231.
5. Kwon SY, Kim TK, Kim YS, Lee KY, Lee NJ, Seol HY. CT findings in Kikuchi disease: analysis of 96 cases. AJNR Am J Neuroradiol. 2004. 25:1099–1102.
6. Turner RR, Martin J, Dorfman RF. Necrotizing lymphadenitis. A study of 30 cases. Am J Surg Pathol. 1983. 7:115–123.
7. Miller WT Jr, Perez-Jaffe LA. Cross-sectional imaging of Kikuchi disease. J Comput Assist Tomogr. 1999. 23:548–551.

8. Pyeun YS, Lee SW, Rho MH. Ultrasonographic evaluation of Kikuchi disease in the neck. J Korean Soc Med Ultrasound. 2001. 20:221–226.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download