Abstract
This study was conducted to evaluate the effects of vardenafil (Levitra), a phosphodiesterase-5 (PDE-5) inhibitor, on cell proliferation in the hippocampal dentate gyrus and on 5-hyroxytryptamine (5-HT, serotonin) synthesis and tryptophan hydroxylase (TPH) expression in the rat dorsal raphe nucleus. Male Sprague-Dawley rats were divided into 6 groups (n=5 in each group): a control group, a 0.5 mg/kg-1 day vardenafil-treated group, a 1 mg/kg-1 day vardenafil-treated group, a 2 mg/kg-1 day vardenafil-treated group, a 1 mg/kg-3 day vardenafil-treated group, and a 1 mg/kg-7 day vardenafil-treated group. 5-bromo-2'-deoxyuridine (BrdU) immunohistochemistry was then performed to evaluate cell proliferation in the dentate gyrus. In addition, 5-HT and TPH immunohistochemistry was conducted to evaluate serotonin expression in the dorsal raphe. The results revealed that treatment with vardenafil increased cell proliferation in the dentate gyrus and enhanced 5-HT synthesis and TPH expression in the dorsal raphe in a dose- and duration-dependent manner. The findings demonstrate that the increasing effect of vardenafil on cell proliferation is closely associated with the enhancing effect of vardenafil on serotonin expression under normal conditions.
Vardenafil (Levitra, Bayer, Monheim, Germany) is a selective phosphodiesterase-5 (PDE-5) inhibitor, and it has been approved for the treatment of erectile dysfunction (1). PDE-5 is implicated in numerous physiological processes, including apoptosis and neurogenesis (2). Many studies have proven that PDE-5 inhibitors are effective for the treatment of various disorders, such as chronic obstructive pulmonary disease, prostatic hyperplasia, hypertension, coronary heart disease, and brain injury (3).
The process of neurogenesis, the birth of new neurons, in the hippocampal dentate gyrus occurs in a variety of mammals, including humans (4, 5). Previous studies have shown that several factors, such as glucocorticoids, estrogen, N-methyl-D-aspartate receptor antagonists, growth factors, serotonin, aging, seizures, and environmental stimuli, influence the proliferation of granule cell precursors and/or neurogenesis in the adult dentate gyrus (4-6).
Serotonin (5-hydroxytryptamine, 5-HT) is an important neurotransmitter and/or neuromodulator in the mammalian central nervous system (CNS). Most cell bodies of serotonergic neurons in the brain arise from the dorsal raphe, and these cells send their projections to diverse target regions, including the olfactory bulb, hypothalamus, septum, striatum, thalamus, caudoputamen, and cerebral cortex. 5-HT may be considered a positive regulatory factor of adult granule cell proliferation and it may facilitate granule cell proliferation in the dentate gyrus (6, 7). Tryptophan hydroxylase (TPH) catalyzes the rate-limiting step of serotonin biosynthesis in the serotonergic neurons of the raphe nuclei, and the enzymatic activity of TPH in the brain regulates 5-HT synthesis during normal development (8).
PDE-5 is highly specific for hydrolysis of cyclic guanosine monophosphate (cGMP) and PDE-5 is a key modulator of the intracellular cGMP signaling pathway. Some studies showed that selective PDE-5 inhibitors affected memory performance in the object recognition task and improved memory consolidation in adult rats (9, 10). Thus, the possibility that PDE-5 inhibitors may regulate angiogenesis, cell proliferation and/or neurogenesis was suggested. However, there was no such investigation for the neuronal cell proliferation related with PDE-5 inhibitor, vardenafil, under normal conditions.
In the present study, we investigated the effects of vardenafil on cell proliferation in the hippocampal dentate gyrus and on 5-HT synthesis and TPH expression in the rat dorsal raphe. For the detection of cell proliferation in the dentate gyrus, 5-bromo-2'-deoxyuridine (BrdU) immunohistochemistry was performed, and 5-HT and TPH immunohistochemistry was conducted for the evaluation of serotonin expression in the dorsal raphe.
Male Sprague-Dawley rats weighing 300±10 g (n=30) were used for the experiment. The animals were housed under controlled temperature (20±2℃) and maintained on lightdark cycles, each consisting of 12 hr of light and 12 hr of darkness (lights on from 07:00 to 19:00). The experimental procedures were performed in accordance with the animal care guidelines of National Institutes of Health (NIH) and Korean Academy of Medical Sciences.
The rats were divided into 6 groups (n=5 in each group): the control group, 0.5 mg/kg-1 day vardenafil-treated group, 1 mg/kg-1 day vardenafil-treated group, 2 mg/kg-1 day vardenafil-treated group, 1 mg/kg-3 day vardenafil-treated group, and 1 mg/kg-7 day vardenafil-treated group.
Vardenafil was obtained from Bayer Pharmaceuticals Corporation (Monheim, Germany) and dissolved in distilled water, acidified with HCl to pH 4.5 as the previously described method (11). The appropriate dose of vardenafil was administered orally (p.o.) to the rats in each experimental group once daily for 7 consecutive days. The animals in the control group received the same amount of distilled water for the same duration. All rats received intraperitoneal injection of BrdU (50 mg/kg, i.p.) once daily for 7 consecutive days at 30 min before the vardenafil administration.
The rats were sacrificed on the 8th day of the experiment. At the beginning of the sacrificial procedure, the animals were weighed and given an overdose of Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). After a complete lack of response was observed, the rats were transcardially perfused with 50 mM phosphate-buffered saline (PBS) and then with 4% paraformaldehyde in 100 mM phosphate buffer (PB) at pH 7.4. The brains were dissected, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Serial coronal sections of 40-µm thickness were made using a freezing microtome (Leica, Nussloch, Germany).
For visualization of cell birth in the hippocampal dentate gyrus, BrdU-specific immunohistochemistry was performed as previously described (12). Briefly, brain sections were permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, incubated in 50% formamide-2×standard saline citrate (SSC) at 65℃ for 2 hr, denatured in 2 N HCl at 37℃ for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). These processes were executed in due order. The sections were then incubated overnight at 4℃ with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannhein, Germany). The sections were washed three times with PBS and incubated for 1 hr with biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, U.S.A.). The sections were incubated for an additional 1 h with avidinbiotin-horseradish-peroxidase complex (1:100; Vector Laboratories). For visualization, the sections were incubated for 5 min in 50 mM Tris-HCl (pH 7.6) containing 0.03% 3,3-diaminobenzidine (DAB; Sigma Chemical Co., St. Louis, MO, U.S.A.), 40 mg/mL nickel chloride, and 0.03% hydrogen peroxide. Subsequently, the slides were air-dried overnight at room temperature, and cover slides were mounted using Permount® (Fisher Scientific, New Jersey, NJ, U.S.A.).
For the detection of 5-HT-positive and TPH-positive cells in the dorsal raphe, immunohistochemistry for the detection of 5-HT and TPH expressions was performed as the previously described method (13). To begin the procedure, five sections on average were selected in each brain region spanning from bregma -7.30 mm to -8.00 mm. The sections were incubated in PBS for 10 min and washed three more times with PBS. The sections were then incubated in 1% hydrogen peroxide (H2O2) for 30 min. The sections were incubated overnight with rabbit anti-5-HT antibody (1:500; Oncogene Research Product, Cambridge, U.K.) in order to visualize 5-HT expression or with mouse monoclonal anti-TPH antibody (1:1,000; Oncogene Research Product) to visualize TPH expression. The sections were then incubated for 1 hr with biotinylated anti-rabbit secondary antibody (1:200; Vector Laboratories) or with anti-mouse secondary antibody (1: 200; Vector Laboratories). The sections were subsequently incubated with avidin-biotin-peroxidase complex (1:100; Vector Laboratories) for 1 hr at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.03% DAB and 0.01% H2O2 in 50 mM Tris-buffer (pH 7.6) for approximately 3 min. The sections were then mounted on gelatin-coated glass slides.
The area of the granular layer of the dentate gyrus was measured using an Image-Pro®Plus image analyzer (Media Cybernetics Inc., Silver Spring, MD, U.S.A.). The number of BrdU-positive cells was counted hemilaterally and expressed as the number of cells per mm2 of the cross-sectional area of the granular layer of the dentate gyrus. The numbers of 5-HT-positive and TPH-positive cells were counted and expressed as the number of cells per mm2 of the cross-sectional area of the dorsal raphe. All data were analyzed using SPSS statistical software (version 12.0). The data were expressed as the mean±standard error of the mean (SEM). For comparisons among the groups, one-way ANOVA and Duncan's post-hoc test were performed, and differences were considered statistically significant at P<0.05.
In order to investigate the dose-dependent effect of vardenafil on cell proliferation, the rats were treated with various doses of vardenafil for 1 day. The number of BrdU-positive cells in the dentate gyrus of the hippocampus was 192.08±20.74/mm2 in the control group, 220.64±14.74/mm2 in the 0.5 mg/kg vardenafil-treated group, 219.53±12.59/mm2 in the 1 mg/kg vardenafil-treated group, and 267.12±20.82/mm2 in the 2 mg/kg vardenafil-treated group.
In order to investigate the duration-dependent effect of vardenafil on cell proliferation, the rats were treated with 1 mg/kg vardenafil during 1 day, 3 day, and 7 day. The number of BrdU-positive cells in the dentate gyrus of the hippocampus was 192.08±20.74/mm2 in the control group, 219.53±12.59/mm2 in the 1 day vardenafil-treated group, 275.27±17.68/mm2 in the 3 day vardenafil-treated group, and 284.64±14.86/mm2 in the 7 day vardenafil-treated group.
These results showed that vardenafil treatment increased cell proliferation in the detate gyrus in the dose- and duration-dependent manner (Fig. 1).
In order to investigate the dose-dependent effect of vardenafil on 5-HT synthesis, the rats were treated with various doses of vardenafil for 1 day. The number of 5-HT-positive cells in the dorsal raphe nucleus was 78.33±4.63 in the control group, 104.55±6.60 in the 0.5 mg/kg vardenafil-treated group, 117.64±5.55 in the 1 mg/kg vardenafil-treated group, and 161.25±6.06 in the 2 mg/kg vardenafil-treated group.
In order to investigate the duration-dependent effect of vardenafil on 5-HT synthesis, the rats were treated with 1 mg/kg vardenafil during 1 day, 3 day, and 7 day. The number of 5-HT-positive cells in the dorsal raphe nucleus was 78.33±4.63 in the control group, 117.64±5.55 in the 1 day vardenafil-treated group, 141.08±4.74 in the 3 day vardenafil-treated group, and 150.64±7.47 in the 7 day vardenafil-treated group.
These results showed that vardenafil treatment increased 5-HT synthesis in the dorsal raphe in the dose- and duration-dependent manner (Fig. 2).
In order to investigate the dose-dependent effect of vardenafil on TPH expression, the rats were treated with various doses of vardenafil for 1 day. The number of TPH-positive cells in the dorsal raphe was 371.67±34.57 in the control group, 378.56±47.39 in the 0.5 mg/kg vardenafil-treated group, 435.36±55.00 in the 1 mg/kg vardenafil-treated group, and 566.50±33.22 in the 2 mg/kg vardenafil-treated group.
In order to investigate the duration-dependent effect of vardenafil on TPH expression, the rats were treated with 1 mg/kg vardenafil during 1 day, 3 day, and 7 day. The number of TPH-positive cells in the dorsal raphe was 371.67±34.57 in the control group, 435.36±55.00 in the 1 day vardenafil-treated group, 576.44±62.74 in the 3 day vardenafil-treated group, and 584.33±34.55 in the 7 day vardenafil-treated group.
These results showed that vardenafil treatment increased TPH expression in the dorsal raphe in the dose- and duration-dependent manner (Fig. 3).
The results of this study demonstrated that vardenafil enhances cell proliferation in the hippocampal dentate gyrus. Vardenafil is considered to be slightly more potent than sildenafil and tadalafil. This enhanced potency may be due to its different chemical structure, which allows it to dissociate from PDE5 more slowly than sildenafil and tadalafil (14). In addition, inhibition of PDE5 activity is known to augment neurogenesis in the subventricular zone of aged rats following focal cerebral ischemia (15-17). Furthermore, Zhang et al. reported that treatment with sildenafil, a PDE5 inhibitor, increased brain levels of cGMP, evoked neurogenesis, and reduced neurological deficits when administered to rats 2 or 24 hr after stroke (16). They also showed that treatment with sildenafil significantly improved functional recovery and increased vascular density, endothelial cell proliferation, and synaptogenesis in aged rats following embolic stroke. In aged stroke rats, improvement of functional recovery by sildenafil is likely fostered by the enhancement of angiogenesis and synaptogenesis (15).
PDE-5 inhibitors have been reported to enhance cell proliferation under ischemic conditions; however, no studies have been conducted to evaluate the effect of vardenafil on cell proliferation under normal conditions. In this study, vardenafil was found to enhance cell proliferation in the dentate gyrus under normal conditions, and these effects were found to occur in a dose- and duration-dependent fashion.
The present results demonstrated that treatment with vardenafil increases 5-HT synthesis and TPH expression in the dorsal raphe. 5-HT has been implicated in hippocampal neurogenesis (18), and Brezun and Daszuta reported that the depletion of serotonin in the brain led to decreased cell proliferation in the subventricular zone and dentate gyrus (19). Enhanced 5-HT synthesis stimulates neurogenesis in the hippocampus via 5-HT receptors, while suppression or lesion of hippocampal 5-HT neurotransmission exerts the opposite effect (20). In addition, the hippocampal dentate gyrus is densely innervated by serotonergic fibers (21) and contains abundant 5-HT1A receptors (22). Therefore, increased serotonin levels in the brain also enhance cell proliferation in the dentate gyrus (23). Furthermore, a reduction in TPH activity is known to rapidly decrease the release of 5-HT, indicating that changes in the level of TPH can profoundly influence 5-HT synaptic activity (24).
The relationship between serotonin expression and cell proliferation has been previously reported; however, there have been no reports of the effect of vardenafil on serotonin expression under normal conditions. The results of the present study demonstrated that vardenafil increases 5-HT synthesis and TPH expression in the dorsal raphe under normal conditions. Furthermore, the effects of vardenafil on 5-HT synthesis and TPH expression were found to occur in a dose- and duration dependant manner.
Increased cell proliferation in the dentate gyrus is known to be involved in memory processing, cognition, and brain repair effects (5, 12, 17), and it has been suggested that serotonin and neurotrophic factors, such as basic fibroblast growth factor (bFGF), insulin-like growth factor-1 (IGF-1), and BDNF induce hippocampal cell proliferation and/or neurogenesis (18, 19, 25, 26).
In this study, we confirm that the effect of vardenafil on cell proliferation is closely associated with the enhancement of serotonin expression in normal rats by vardenafil. Decreased cell proliferation is closely associated with age-dependent memory decline and stressful conditions (27). Decreased serotonin expression is implicated in depressive disorders (28). These results suggest that the enhancement of cell proliferation by vardenafil may be modulated by the activity of serotonergic neurons under normal conditions. Based on the present results, it is possible that vardenafil can be used to stimulate neuronal function by stimulating cell proliferation in the CNS disorders with hippocampal memory impairment through enhancing of the serotonergic nerve system.
Figures and Tables
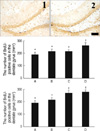 | Fig. 1Effect of vardenafil treatment on cell proliferation in the dentate gyrus. Above: Photomicrographs of 5-bromo-2'-deoxyuridine (BrdU)-positive cells in the dentate gyrus. 1. Control group, 2. 2 mg/kg vardenafil for 1 day-treated group. The scale bar represents 50 µm. Middle: The dose-dependent effect of vardenafil treatment for 1 day on cell proliferation in the dentate gyrus. (A) Control group, (B) 0.5 mg/kg vardenafil-treated group, (C) 1 mg/kg vardenafil-treated group, (D) 2 mg/kg vardenafil-treated group. Lower: The duration-dependent effect of 1 mg/kg vardenafil treatment on cell proliferation in the dentate gyrus. (A) Control group, (B) 1-day vardenafil-treated group, (C) 3-day vardenafil-treated group, (D) 7-day vardenafil-treated group. Values are represented as the mean±SEM. |
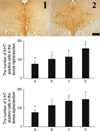 | Fig. 2Effect of vardenafil treatment on 5-hyroxytryptamine (5-HT) expression in the dorsal raphe nucleus. Upper: Photomicrographs of 5-HT-positive cells in the dorsal raphe nucleus. 1. Control group, 2. 2 mg/kg vardenafil for 1 day-treated group. The scale bar represents 100 µm. Middle: The dose-dependent effect of 1-day vardenafil treatment on 5-HT expression in the dorsal raphe nucleus. (A) Control group, (B) 0.5 mg/kg vardenafil-treated group, (C) 1 mg/kg vardenafil-treated group, (D) 2 mg/kg vardenafil-treated group. Lower: The duration-dependent effect of 1 mg/kg vardenafil treatment on 5-HT expression in the dorsal raphe nucleus. (A) Control group, (B) 1-day vardenafil-treated group, (C) 3-day vardenafil-treated group, (D) 7-day vardenafil-treated group. Values are represented as the mean±SEM. |
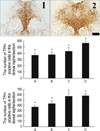 | Fig. 3Upper: Photomicrographs of tryptophan hydroxylase (TPH)-positive cells in the dorsal raphe nucleus. 1. Control group, 2. 2 mg/kg vardenafil for 1 day-treated group. The scale bar represents 100 mm. Middle: The dose-dependent effect of 1-day vardenafil treatment on TPH expression in the dorsal raphe nucleus. (A) Control group, (B) 0.5 mg/kg vardenafil-treated group, (C) 1 mg/kg vardenafil-treated group, (D) 2 mg/kg vardenafil-treated group. Lower: The duration-dependent effect of 1 mg/kg vardenafil treatment on TPH expression in the dorsal raphe nucleus. (A) Control group, (B) 1-day vardenafil-treated group, (C) 3-day vardenafil-treated group, (D) 7-day vardenafil-treated group. Values are represented as the mean±SEM. |
References
1. Reffelmann T, Kloner RA. Vardenafil: a selective inhibitor of phosphodiesterase-5 for the treatment of erectile dysfunction. Expert Opin Pharmacother. 2007. 8:965–974.

2. Lin CS, Lin G, Xin ZC, Lue TF. Expression, distribution and regulation of phosphodiesterase 5. Curr Pharm Des. 2006. 12:3439–3457.

3. van Driel MF. Phosphodiesterase inhibitors: effectiveness and new applications. Ned Tijdschr Geneeskd. 2006. 150:1613–1616.
4. Eriksson PS, Perfilieva E, Björk-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998. 4:1313–1317.

5. Fuchs E, Gould E. Mini-review: in vivo neurogenesis in the adult brain: regulation and functional implications. Eur J Neurosci. 2000. 12:2211–2214.
6. Djavadian RL. Serotonin and neurogenesis in the hippocampal dentate gyrus of adult mammals. Acta Neurobiol Exp (Wars). 2004. 64:189–200.
7. Brezun JM, Daszuta A. Serotoinin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur J Neurosci. 2000. 12:391–396.
8. Rind HB, Russo AF, Whittemore SR. Developmental regulation of tryptophan hydroxylase messenger RNA expression and enzyme activity in the raphe and its target fields. Neuroscience. 2000. 101:665–677.

9. Prickaerts J, Sik A, van der Staay FJ, de Vente J, Blokland A. Dissociable effects of acetylcholinesterase inhibitors and phosphodiesterase type 5 inhibitors on object recognition memory: acquisition versus consolidation. Psychopharmacology (Berl). 2005. 177:381–390.

10. Rutten K, Basile JL, Prickaerts J, Blokland A, Vivian JA. Selective PDE inhibitors rolipram and sildenafil improve object retrieval performance in adult cynomolgus macaques. Psychopharmacology (Berl). 2008. 196:643–648.

11. Ferrini MG, Kovanecz I, Nolazco G, Rajfer J, Gonzalez-Cadavid NF. Effects of long-term vardenafil treatment on the development of fibrotic plaques in a rat model of Peyronie's disease. BJU Int. 2006. 97:625–633.

12. Kim H, Lee SH, Kim SS, Yoo JH, Kim CJ. The influence of maternal treadmill running during pregnancy on short-term memory and hippocampal cell survival in rat pups. Int J Dev Neurosci. 2007. 25:243–249.

13. Kim EK, Lee MH, Kim H, Sim YJ, Shin MS, Lee SJ, Yang HY, Chang HK, Lee TH, Jang MH, Shin MC, Lee HH, Kim CJ. Maternal ethanol administration inhibits 5-hydroxytryptamine synthesis and tryptophan hydroxylase expression in the dorsal raphe of rat offspring. Brain Dev. 2005. 27:472–476.

14. Blount MA, Beasley A, Zoraghi R, Sekhar KR, Bessay EP, Francis SH, Corbin JD. Binding of tritiated sildenafil, tadalafil, or vardenafil to the phosphodiesterase-5 catalytic site displays potency, specificity, heterogeneity, and cGMP stimulation. Mol Pharmacol. 2004. 66:144–152.

15. Zhang L, Zhang RL, Wang Y, Zhang C, Zhang ZG, Meng H, Chopp M. Functional recovery in aged and young rats after embolic stroke: treatment with a phosphodiesterase type 5 inhibitor. Stroke. 2005. 36:847–852.
16. Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Zhang L, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002. 33:2675–2680.

17. Zhang RL, Zhang Z, Zhang L, Wang Y, Zhang C, Chopp M. Delayed treatment with sildenafil enhances neurogenesis and improves functional recovery in aged rats after focal cerebral ischemia. J Neurosci Res. 2006. 83:1213–1219.

18. Jha S, Rajendran R, Davda J, Vaidya VA. Selective serotonin depletion does not regulate hippocampal neurogenesis in the adult rat brain: differential effects of p-chlorophenylalanine and 5,7-dihydroxytryptamine. Brain Res. 2006. 1075:48–59.

19. Brezun JM, Daszuta A. Depletion in serotonin decreases neurogenesis in the dentate gyrus and the subventricular zone of adult rats. Neuroscience. 1999. 89:999–1002.

20. Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004. 29:450–460.

21. Nishimura A, Ueda S, Takeuchi Y, Sawada T, Kawata M. Age-related decrease of serotonergic fibres and S-100 beta immunoreactivity in the rat dentate gyrus. Neuroreport. 1995. 6:1445–1448.
22. Pompeiano M, Palacios JM, Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain correlation with receptor binding. J Neurosci. 1992. 12:440–453.
23. Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000. 20:9104–9110.

24. Gartside SE, Cowen PJ, Sharp T. Evidence that the large neutral amino acid L-valine decreases electrically-evoked release of 5-HT in rat hippocampus in vivo. Psychopharmacology (Berl). 1992. 109:251–253.
25. Gomez-Pinilla F, So V, Kesslak JP. Spatial learning and physical activity contribute to the induction of fibroblast growth factor: neural substrates for increased cognition associated with exercise. Neuroscience. 1998. 85:53–61.

26. Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001. 21:5678–5684.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download