Abstract
Lindera erythrocarpa Makino (Lauraceae) is used as a traditional medicine for analgesic, antidote, and antibacterial purposes and shows anti-tumor activity. We studied the effects of Lindera erythrocarpa on the human ether-a-go-go-related gene (HERG) channel, which appears of importance in favoring cancer progression in vivo and determining cardiac action potential duration. Application of MeOH extract of Lindera erythrocarpa showed a dose-dependent decrease in the amplitudes of the outward currents measured at the end of the pulse (IHERG) and the tail currents of HERG (Itail). When the BuOH fraction and H2O fraction of Lindera erythrocarpa were added to the perfusate, both IHERG and Itail were suppressed, while the hexane fraction, CHCl3 fraction, and EtOAc fraction did not inhibit either IHERG or Itail. The potential required for half-maximal activation caused by EtOAc fraction, BuOH fraction, and H2O fraction shifted significantly. The BuOH fraction and H2O fraction (100 µg/mL) decreased gmax by 59.6% and 52.9%, respectively. The H2O fraction- and BuOH fraction-induced blockades of Itail progressively decreased with increasing depolarization, showing the voltage-dependent block. Our findings suggest that Lindera erythrocarpa, a traditional medicine, blocks HERG channel, which could contribute to its anticancer and cardiac arrhythmogenic effect.
Lindera erythrocarpa Makino (L. erythrocarpa) belongs to the large family Lauraceae, which consists mostly of trees or shrubs from the warmer regions of the earth. It is distributed throughout Korea, China, and Japan (1). Lindera species, including L. lucida, L. strychnifolia, L. aggregate and L. chunii are important medicinal plants. The fruit of L. erythrocarpa is used as a traditional medicine for analgesic, digestive, diuretic, anti-dote, and antibacterial purposes; also, its leaves have been used as a folk medicine for stomach-ache, thirst, and neuralgia (1-3). Cyclopentenediones, farnesyl protein transferase inhibitors, and anti-tumor compounds were isolated from the methanolic extract of the fruits of L. erythrocarpa (4). These compounds strongly inhibit human colon tumor cells and exert their anti-tumor activity by inducing apoptosis through the caspase-3 pathway (4). Also, three lignans isolated from a methanol extract of L. erythrocarpa were evaluated for in vitro cytotoxicity using three cancer cell line assays, and among these compounds, methyllinderone showed significant cytotoxicity against mouse melanoma, human acetabulum fibrosarcoma, and myelogenous leukemia cell lines (5).
The rapid component of a cardiac delayed rectifier potassium current (IKr) is known to play a critical role in repolarization of action potential (6). IKr is one of the targets for antiarrhythmic therapy, since the blocking of this current is expected to increase the action potential duration (APD) and thereby increase the refractory period (7). It has been shown that the human ether-a-go-go-related gene (HERG) encodes a major component of the IKr and that congenital mutation of HERG results in long QT syndrome, which shows a high risk of sudden death due to torsade de pointes type ventricular arrhythmia (8). While HERG K+ channels control electrical activity in excitable cells, these channels are also significantly expressed in non-excitable cells. High levels of HERG mRNA are detected in various cancer cells (9-10), and the overexpression of ether-a-go-go gene (EAG) channels enhances aggressive growth of tumors (11). Moreover, E-4031, a specific HERG channel blocker, reduced proliferation of uterine cancer cells (12). Therefore, the cytotoxic effect of L. erythrocarpa for the cancer cell lines could be caused by the modulation of HERG K+ channels (5).
In the present study, we have investigated the effect of extracts of L. erythrocarpa on the HERG current, a molecular equivalent of IKr, using the Xenopus oocyte expression system. We found that L. erythrocarpa blocked the HERG channel, resulting in a shift in voltage-dependence of channel activation and reduction of maximum conductance (gmax). We have also examined the HERG-blocking effects of several fractions of L. erythrocarpa, and the H2O and BuOH fractions exhibited the strongest changes in V1/2 (the potential required for half-maximal activation) and gmax (maximum conductance) among the solvent fractions.
The leaves of L. erythrocarpa were collected in October 2005 at Jeju Island, Korea. The samples were cleaned, dried at room temperature for two weeks, and ground into a fine powder. The dried materials (100 g) were extracted with 80% methanol (MeOH) at room temperature for 24 hr and then concentrated under a vacuum. The resulting MeOH extract (32 g) was suspended in water (1 L) and successively partitioned with hexane (1 L×3), chloroform (CHCl3; 1 L×3) ethyl acetate (EtOAc; 1 L×3), and n-butanol (BuOH; 1 L×3), to give hexane (0.9892 g), CHCl3 (1.6209 g), EtOAc (3.0058 g), BuOH (6.8221 g) and H2O (18.5571 g) fractions, respectively.
Complementary HERG (accession no. U04270) RNA was synthesized by in vitro transcription from 1 µg of linearized cDNA using T7 message machine kits (Ambion, Austin, TX, U.S.A.) and stored in 10 mM Tris-HCl (pH 7.4) at -80℃. Stage V-VI oocytes were surgically removed from female Xenopus laevis (Nasco, Modesto, CA, U.S.A.) that was anesthetized with 0.17% tricane methanesulphonate (Sigma Chemicals, St. Louis, MO, U.S.A.). Using fine forceps, theca and follicle layers were manually removed from oocytes, which were injected with 40 nL of cRNA (0.1-0.5 µg/µL). The injected oocytes were maintained in modified Barth's solution containing 88 mM NaCl, 1 mM KCl, 0.4 mM CaCl2, 0.33 mM Ca (NO3)2, 1 mM MgSO4, 2.4 mM NaHCO3, 10 mM HEPES (pH 7.4), and 50 µg/mL gentamicin sulphonate. Currents were studied two to seven days after injection.
Normal Ringer's solution contained 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 10 mM HEPES (pH adjusted to 7.4 with NaOH). All salts were purchased from Sigma Chemicals. The effects of the MeOH extract and solvent fractions on the HERG current were observed by adding 100 mg/mL stock solution of either MeOH extract or solvent fractions to the external solutions at suitable concentrations (0.01-300 µg/mL) shortly before each experiment. None of the final concentrations from any of the solvents exceeded 0.1%. Solutions were applied to the oocytes by continuous perfusion of the chamber while recording. Solution exchanges were completed within 3 min, and the HERG current was recorded after 5 min when the solution exchange was completed. The effects of several concentrations of MeOH extract, BuOH fraction, and H2O fraction on the HERG currents were determined after the currents showed reversibility when washed with normal Ringer's solution. It took about 10 min to wash out ≤30 µg/mL MeOH extract or BuOH and H2O fractions, and about 20 min to wash out ≥50 µg/mL. In experiments with 50-100 µg/mL BuOH and H2O fractions, HERG currents usually (-80% of cells examined) showed >90% recovery after 20 min washing with normal Ringer's solution. If the oocyte did not recover current after 30 min of washing with normal Ringer's solution, it was not used further. Currents were measured at room temperature (21-23℃) with a two-microelectrode voltage clamp amplifier (Warner Instruments, Hamden, CT, U.S.A.). Electrodes were filled with 3 M KCl and had a resistance of 2-4 MΩ for voltage-recording electrodes and 0.6-1 MΩ for current-passing electrodes. Stimulation and data acquisition were controlled with Digidata and pCLAMP software (Axon Instruments, Sunnyvale, CA, U.S.A.).
We assayed the effects of MeOH extract and solvent fractions of L. erythrocarpa on HERG current using a Xenopus oocyte expression system. Throughout the experiments, the holding potential was held at -70 mV, and the repolarization potential was constantly held at -60 mV for the analysis of tail currents (Itail). Fig. 1A shows an example of a voltage-clamp recording from a Xenopus oocyte and representative current traces for control conditions and after exposure to 100 µg/mL MeOH extract of L. erythrocarpa. Under control conditions, depolarizing steps activated time-dependent outward currents. The amplitude of the outward currents measured at the end of the pulse (IHERG) increased with more positive voltage steps, reaching a maximum value at 0 mV. Depolarizing steps to even more positive values caused a current decrease, resulting in a negative slope of the I-V curve (Fig. 1B). Current-voltage relationships for IHERG obtained at various concentrations of L. erythrocarpa extract are plotted in Fig. 1B. As the concentration of the extract progressively increased, the amplitude of IHERG showed a dose-dependent decrease.
After the depolarizing steps, repolarization to -60 mV induced outward Itail, which had an amplitude even larger than that of IHERG during depolarization. This is a characteristic property of HERG currents, which is due to rapid recovery from inactivation and a slow deactivation mechanism (13). The amplitude of Itail increased with depolarizing steps from -50 to +20 mV, and was then superimposed on further depolarizing steps to +40 mV. When 100 µg/mL MeOH extract of L. erythrocarpa was added to the perfusate, both IHERG and Itail were suppressed (Fig. 1A, bottom panel). The amplitude of Itail was normalized to the peak amplitude obtained under control conditions at maximum depolarization and was plotted against the potential of the step depolarization (Fig. 1C). The normalized Itail reflects a voltage-dependent activation of the HERG channels. Data obtained under control conditions were well fitted by the Boltzmann equation, with half-maximal activation (V1/2) at -15.2 mV. As the concentration of the MeOH extract of L. erythrocarpa was increased, the peak Itail amplitude decreased, indicating that the gmax of HERG channels is decreased by L. erythrocarpa.
For further analysis, we normalized the values in Fig. 1C to the respective maximum value at each concentration to examine a possible activation curve shift induced by the MeOH extract of L. erythrocarpa. As shown in Fig. 1D, the activation curves at control, 10, 20, and 100 µg/mL of MeOH extract are shifted to the right according to increases in concentration, while the activation curves at 200 and 300 µg/mL MeOH extract basically overlapped. The V1/2 calculations were consistent with this finding, yielding values of -15.2±0.42, -14.2±0.28, -8.36±0.35, 3.21±0.69, 1.10±0.59, -3.85±1.97, and -2.38±0.83 mV at control, 10, 20, 50, 100, 200 and 300 µg/mL MeOH extract, respectively (n=8). Thus, the V1/2 values for experiments run in the presence of 10-100 µg/mL MeOH extract significantly increased according to concentration (P<0.05, n=8), whereas those for experiments run in the presence of 200 and 300 µg/mL, relatively high concentrations, are not significantly different from each other (P>0.05, n=8). These findings indicate that MeOH extract of L. erythrocarpa may change activation gating at 10-100 µg/mL, while those high concentrations such as 200 and 300 µg/mL have induced maximal effects on HERG channel current.
In addition, the effect of MeOH extract of L. erythrocarpa on activated currents was tested. The activated current-voltage relationship was obtained with the use of a voltage-clamp protocol shown at the top of Fig. 2A. A depolarizing pulse to +50 mV, which induced a full activation, was followed by various levels of test pulses. The amplitude of the current was measured at its peak before deactivation occurred, and then the data were normalized to the respective maximal currents in the control. Next, the normalized data were averaged and plotted against the test potential (Fig. 2B). The activated current-voltage relationship showed a typical inward rectification of the HERG channel due to the rapid inactivation. The MeOH extract of L. erythrocarpa reduced the current within the wide range of membrane potentials from -140 to +30 mV concentration-dependently. Also, the reversal potentials before and after treatment with 10, 100, and 300 µg/mL MeOH extract were -91.3±1.9, -90.5±2.3 (P>0.05, n=9), -85.8±2.4, and -77.5±3.2 mV, respectively (P<0.05, n=9). Considering that K+ channel has relatively high selectivity for the permeating ion, K+ rather than Na+, Ca2+, and Cl-, the MeOH extract at relatively high concentrations of 100 and 300 µg/mL, could perturb the ion-selectivity of HERG channel by inducing non-specific membrane permeabilization or membrane leakage.
Next, we examined the effect of several solvent fractions of L. erythrocarpa on the HERG current by comparing the changes of IHERG and Itail by each solvent fraction, using the hexane fraction, CHCl3 fraction, EtOAc fraction, BuOH fraction, and H2O fraction at the same concentrations (100 µg/mL) using the same protocol as Fig. 1, 3A shows an example of a voltage-clamp recording from the Xenopus oocyte cell with the representative current traces from a cell in control and after exposure to 100 µg/mL of each solvent fraction. When 100 µg/mL of the hexane fraction, CHCl3 fraction, or EtOAc fraction of L. erythrocarpa was added to the perfusate, both IHERG and Itail were suppressed relatively weakly (Fig. 3A, upper and lower panels). In contrast, 100 µg/mL of the BuOH fraction or the H2O fraction inhibited both IHERG and Itail relatively strongly (Fig. 3A, lower panels). The amplitudes of IHERG and Itail were normalized to the peak amplitude obtained under control conditions and were plotted against the potential of the step depolarization (Fig. 3B, C, respectively). Then, we normalized values in Fig. 3C to the respective maximum value at each fraction to examine possible activation curve shifts induced by fractions of L. erythrocarpa. As shown in Fig. 3D, the activation curves in the control oocytes, as well as those treated with the hexane fraction and CHCl3 fraction, basically overlapped, whereas the curves for the EtOAc fraction, BuOH fraction, and H2O fraction were shifted rightward. The V1/2 calculations were consistent with this finding, yielding values of -15.3±0.43, -17.0±0.47, -14.1±0.20, -8.37±0.94, -2.77±2.12, and 0.56±0.64 mV in the control and the hexane fraction, CHCl3 fraction, EtOAc fraction, BuOH fraction and H2O fraction-treated groups, respectively (n=8-22). These findings indicate that the EtOAc fraction, BuOH fraction, and H2O fraction of L. erythrocarpa may change activation gating at 100 µg/mL.
We then compared the effects of L. erythrocarpa fractions on values of V1/2, and the gmax that is obtained from the maximal amplitude of the Itail curve in Fig. 3C. As shown in Table 1, the BuOH fraction and H2O fraction changed V1/2 significantly (n=8, P<0.05) and decreased gmax by 59.6±8.7% and 52.9±3.5%, respectively (n=8, P<0.05), while EtOAc fraction changed V1/2 significantly (n=8, P<0.05) but did not change gmax (n=8, P>0.05).
Since the H2O and BuOH fractions exhibited the strongest changes in V1/2 and gmax among the solvent fractions, we first determined whether the effect of the H2O fraction of L. erythrocarpa is voltage dependent by comparing the decrease of Itail induced by the fraction at different potentials (Fig. 4). We found that a higher degree of blockade was present at more negative voltages (Fig. 4A). As shown in Fig. 4B, the Itail inhibition by 30 µg/mL of the H2O fraction at different voltages (-40, -20, 0, +20, and +40 mV) was 54.8±8.4, 45.3±3.6, 28.4±1.2, 14.0±1.8, and 9.8±2.7%, respectively (n=6, P<0.05). Dose-response relationships were obtained at -30, 0, and +30 mV, and the data were fitted by Hill equations (Fig. 4C), giving IC50 values of 30.1±1.13, 51.9±3.87, and 83.0±7.80 µg/mL, and Hill coefficients of 1.72±0.11, 1.78±0.20, and 2.13±0.49 at -30, 0 and +30 mV, respectively (n=6), suggesting that the H2O fraction-induced blockade of Itail progressively decreases with increasing depolarization. Collectively, these findings indicate that the H2O fraction of L. erythrocarpa-induced blockade of Itail is voltage-dependent.
Next, we found that a higher degree of blockade by the BuOH fraction of L. erythrocarpa was also present at more negative voltages (Fig. 5). As shown in Fig. 5B, the Itail inhibition by 30 µg/mL BuOH fraction at different voltages (-40, -20, 0, +20, and +40 mV) was 79.7±8.2, 73.0±10.0, 63.2±10.6, 52.4±10.1, and 46.5±10.2%, respectively (n=6, P<0.05). Dose-response relationships were obtained at -30, 0, and +30 mV, and the data were fitted by Hill equations (Fig. 5C), giving IC50 values of 10.4±0.71, 23.4±2.43, and 39.5±7.83 µg/mL, and Hill coefficients of 5.97±10.7, 1.39±0.19, and 1.51±0.42 at -30, 0 and +30 mV, respectively (n=6), suggesting that the BuOH fraction-induced blockade of Itail progressively decreases with increasing depolarization. Collectively, these findings indicate that the BuOH fraction of L. erythrocarpa-induced blockade of Itail is voltage-dependent.
In addition, we found that the block of HERG currents by H2O fraction and BuOH fraction of L. erythrocarpa showed relatively weak time dependence. We activated currents using a protocol containing a single depolarizing step to 0 mV for 8 sec (Fig. 6A). After obtaining the control measurement, 100 µg/mL of the H2O fraction was applied while holding the channels in the closed state at -70 mV and the recordings were made. Analysis of the test pulse after H2O fraction application showed an increase of blocking to 74% at 8 sec in a representative cell (Fig. 6A). The fractional sustained current (obtained by normalizing the currents with H2O fraction of L. erythrocarpa relative to control currents) decreased slightly with ongoing depolarization (Fig. 6B). At the beginning of the pulse, the fractional current was 0.378±0.065 of the control, and it declined slightly to 0.259±0.088 after 1 sec at a test potential of 0 mV (Fig. 6B; n=5). These findings indicate that HERG channels are greatly blocked by H2O fraction of L. erythrocarpa at the holding potential. Similar to the results obtained from the H2O fraction, the BuOH fraction of L. erythrocarpa also shows relatively weak time-dependent block of HERG current (Fig. 6C, D). In a representative cell, 100 µg/mL BuOH fraction application showed an increase of blocking to 79% at 8 sec, and the fractional sustained current slightly decreased with ongoing depolarization (Fig. 6C). At the beginning of the pulse, the fractional current was 0.349±0.052 of the control, and it declined slightly to 0.246± 0.067 after 1 sec at a test potential of 0 mV (Fig. 6D; n=5). These findings indicate that HERG channels are also greatly blocked by BuOH fraction of L. erythrocarpa while remaining at the holding potential.
Our results indicate that extracts of L. erythrocarpa is an inhibitor of HERG channels. The IC50 values of L. erythrocarpa were -50 µg/mL (at +40 mV) for HERG channels heterologously expressed in Xenopus oocytes. Due to the specific properties of the Xenopus oocyte expression system, such as the vitelline membrane and egg yolk, higher extracellular concentrations of the drug are required to block HERG channels than to inhibit native IKr in mammalian cells (14, 15).
It has been shown that various K+ currents are associated with the proliferation of cancer cells. Ca2+-activated K+ channels has been shown to play a role in the physiological regulation during proliferation of human prostate, neuroblastoma or medulloblastoma cancer cells (16-18). The delayed rectifier K+ currents (IK) have been shown to correlated with mitogenesis and to constitute an link between cytokines activated signaling cascade and cell cycle (19, 20). HERG has been found abundantly expressed in a variety of tumor cells of different histogenesis where it plays a crucial role in the development and maintenance of their neoplastic phenotype (21). The high frequency of expression of HERG in primary human endometrial cancers, as compared to normal and hyperplastic endometrium, has candidated this protein as a potential marker of cancerous versus hyperplastic endometrial growth (10). Several models have been proposed to explain the involvement of K+ channels in mitogenesis. Cone and Tonigier have suggested that K+ channel activity would determine a shift in the resting membrane potential of cells, this latter event being mitogenic (22). Since the fruit and leaves of L. erythrocarpa show anti-tumor activity (4, 5), our results suggest that L. erythrocarpa-induced block of the HERG channel could change the resting membrane potential, which may contribute to the anti-tumor effects in various cancer cell lines.
The use of L. erythrocarpa as an antimicrobial or anticancer agent has been suggested. It has been shown that the extract from L. erythrocarpa showed over 90% antimicrobial activity, according to an in vivo bioassay method (23). Lee et al. reported that methyllinderone, one of the compounds isolated from a EtOAc fraction of L. erythrocarpa methanol extract, showed significant cytotoxicity against three cancer cell lines with ED50 values of 2.2-8.3 µg/mL (5). Considering that H2O and BuOH fractions had relatively higher effects on V1/2 and gmax than EtOAc fraction, compounds other than methyllinderone could block the HERG channel.
HERG channel blockers have been shown to inhibit the channels in a voltage-dependent manner, suggesting that these drugs bind to the open or inactivated state of HERG channels. For example, haloperidol, an antipsychotic drug (24), and two histamine receptor antagonists, terfenadine and astemizole (25), have been found to preferentially bind to the inactivated state of HERG, whereas the gastrointestinal prokinetic agent cisapride has been shown to block the channel in its open state (26). We have shown here that the amplitudes of maximum IHERG and the maximum Itail were decreased by L. erythrocarpa extract, and the blockade of Itail magnitude by BuOH and H2O fractions increased with more negative voltages, which decrease the open probability and inhibit inactivation. Also, the voltage dependence of the HERG channel blockade by the BuOH (Fig. 5) and H2O fractions (Fig. 4) indicate that this drug preferentially blocks HERG channels in the closed state rather than either the open or inactivated states. Consistent results were that L. erythrocarpa blocks HERG channel at the holding potential rather than at the depolarizing pulse (Fig. 6). Considering that the V1/2 values of activation curves in the absence and presence of 10-100 µg/mL L. erythrocarpa extract significantly increased according to concentration, our findings suggest that L. erythrocarpa blocks closed state HERG channels and concomitantly changes activation gating. In addition, the shift in V1/2 and the reduction in gmax were greatly affected by the BuOH and H2O fractions rather than the hexane fraction, CHCl3 fraction, or EtOAc fraction (Fig. 3, Table 1). This finding suggested that the active ingredients inhibiting HERG channel should be included in more polar solvent than non-polar solvent.
Shifting of the voltage dependence of activation (Fig. 1D) by positively charged molecule could be considered to be a general phenomenon caused by a non-specific surface charge effect (27, 28). This effect is thought to reflect the binding of the molecule to diffuse or aggregate negatively charged moieties near the outer mouth of the channels, altering the voltage field that is sensed by the channel gating mechanism. According to this hypothesis, current activation and deactivation should be affected to the same extent by L. erythrocarpa. However, there was a larger increase of activation time constant than the decrease of deactivation time constant (n=3, data not shown). In addition, the gmax of HERG channels (current magnitude at highly depolarized potentials where there is already a high probability of opening) was significantly reduced by L. erythrocarpa (Fig. 1C), which cannot be explained by the surface charge theory. Therefore, we excluded the surface charge theory as a main mechanism of the L. erythrocarpa effect.
There are several studies showing the effect of Lindera species (Lauraceae) on K+ channels. Wang et al. (29) suggested that the cardioprotective effect by L. strychnifolia was due to the opening of the mitochondrial KATP channels in cardiac myocytes, because the improvement of myocardial dysfunction after global ischemia by L. strychnifolia was completely abolished by pre-treatment with a mitochondrial KATP channel blocker, 5-HD. In rat isolated ventricular myocytes, dicentrine, an aporphine alkaloid isolated from the root of L. megaphylla, prolongs the APD50 and reduces the rate of action potential upstroke, indicating that dicentrine may possess anti-arrhythmic activity (30). Also, it has been shown that the IK in guinea pig ventricular myocytes was inhibited by dicentrine, with IC50 values between 3 and 10 µM (30). Although it has not been reported that L. erythrocarpa prolongs the APD or induces cardiac arrhythmia to our knowledge, our results suggest that L. erythrocarpa blocks the HERG channel, a major component of the IKr, which could make the heart prone to arrhythmia since IKr is one of the targets for antiarrhythmic therapy and the blocking of this current is expected to increase the APD and thereby increase the refractory period (7).
In conclusion, we have shown here that L. erythrocarpa blocks HERG channel, resulting in a shift of voltage-dependence of channel activation and reduction of gmax, and that the H2O and BuOH fractions exhibits the strongest changes in V1/2 and gmax among the solvent fractions. Considering that L. erythrocarpa have been used as a folk medicine and shows anticancer activity and that HERG channel is important target regulating proliferation of the tumor cells and determining cardiac APD, our results could explain the anticancer effect and potentially arrhythmogenic effect of L. erythrocarpa.
Figures and Tables
Fig. 1
Effect of Lindera erythrocarpa on human-ether-a-go-go-related-gene (HERG) currents elicited by depolarizing voltage pulses. (A) Superimposed current traces elicited by depolarizing voltage pulses (4 sec) in 10 mV steps (upper panel) from a holding potential of -70 mV in the absence of L. erythrocarpa extract (control, middle panel) and in the presence of 100 µg/mL L. erythrocarpa extract (lower panel). (B) Plot of the HERG current (IHERG) measured at the end of depolarizing pulses against the pulse potential in different concentrations of L. erythrocarpa extract (obtained from A). (C) Plot of the normalized tail current measured at its peak just after repolarization. The amplitude of the tail current in the absence of L. erythrocarpa extract was taken as one. Control data were fitted to the Boltzmann equation, y=1/{1+exp [(-V+V1/2)/dx]}, with V1/2 of -15.2 mV. (D) Activation curves with values normalized to the respective maximum value at each concentration of L. erythrocarpa extract. Symbols with error bars represent means±SEM (n=8).

Fig. 2
Effect of L. erythrocarpa extract on the activated current-voltage relationship. (A) Superimposed current traces elicited by various levels of test pulses ranging from -140 to +50 mV following the pre-pulse to +50 mV for 1.5 sec ("inactivation" voltage-clamp protocol, upper panel) before and after the application of 100 µg/mL L. erythrocarpa extract (center and lower panels, respectively). (B) The I-V curve is for the maximal repolarization-evoked (tail) outward currents against the repolarization potential. Symbols with error bars represent the means±S.E.M. (n=9).

Fig. 3
Effect of different solvent fractions of L. erythrocarpa on the HERG current by comparing the changes of IHERG and tail currents with each solvent fraction: the hexane fraction, CHCl3 fraction, EtOAc fraction, BuOH fraction, and H2O fraction at the same concentrations (100 µg/mL). (A) Superimposed current traces from a cell depolarized to +30 mV before and after exposure to the hexane fraction, CHCl3 fraction, EtOAc fraction, BuOH fraction, and H2O fraction of L. erythrocarpa, respectively. (B) Plot of the HERG current (IHERG) measured at the end of depolarizing pulses against the pulse potential in different solvent fractions of L. erythrocarpa (obtained from A). (C) Plot of the normalized tail current measured at its peak just after repolarization. The amplitude of the tail current in the absence of L. erythrocarpa was taken as one. Control data were fitted to the Boltzmann equation, y=1/{1+exp[(-V+V1/2)/dx]}, with V1/2 of -15.3 mV. (D) Activation curves with values normalized to the respective maximum value for each fraction of L. erythrocarpa. Symbols with error bars represent means±SEM (n=8-22).

Fig. 4
Voltage dependence of HERG current blocked by the H2O fraction of L. erythrocarpa. (A) Current traces from a cell depolarized to -40 mV (left panel), 0 mV (middle panel) and +40 mV (right panel), before and after exposure to 30 µg/mL of the H2O fraction of L. erythrocarpa, showing increased blockade of HERG current at the more negative potential. The protocol consisted of 4-sec depolarizing steps to -40 mV, 0 mV or +40 mV from a holding potential of -70 mV, followed by repolarization to -60 mV. (B) HERG current inhibition at different voltages by the H2O fraction of L. erythrocarpa. At each depolarizing voltage step (-40, -20, 0, +20 or +40 mV), the tail currents in the presence of 30 µg/mL H2O fraction of L. erythrocarpa were normalized to the tail current obtained in the absence of L. erythrocarpa. Bars with error bars represent means±SEM (n=6). (C) Concentration-dependent block of HERG current by the H2O fraction at different membrane potentials. At each depolarizing voltage step (-30 mV, 0 mV or +30 mV), the tail currents in the presence of various concentrations of the H2O fraction were normalized to the tail current obtained in the absence of L. erythrocarpa, and plotted against H2O fraction concentrations. Symbols with error bars represent means±SEM (n=6). The line represents the data fits to the Hill equation.
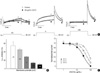
Fig. 5
Voltage dependence of HERG current blocked by the BuOH fraction of L. erythrocarpa. (A) Current traces from a cell depolarized to -40 mV (left panel), 0 mV (middle panel) and +40 mV (right panel), before and after exposure to 30 µg/mL of the BuOH fraction of L. erythrocarpa, showing an increased blockade of HERG current at the more negative potential. The protocol consisted of 4-sec depolarizing steps to -40 mV, 0 mV or +40 mV from a holding potential of -70 mV, followed by repolarization to -60 mV. (B) HERG current inhibition at different voltages by the BuOH fraction of L. erythrocarpa. At each depolarizing voltage step (-40, -20, 0, +20 or +40 mV), the tail currents in the presence of 30 µg/mL BuOH fraction of L. erythrocarpa were normalized to the tail current obtained in the absence of L. erythrocarpa. Bars with error bars represent means±SEM (n=6). (C) Concentration-dependent block of HERG current by the BuOH fraction at different membrane potentials. At each depolarizing voltage step (-30 mV, 0 mV or +30 mV), the tail currents in the presence of various concentrations of the BuOH fraction were normalized to the tail current obtained in the absence of L. erythrocarpa, and plotted against BuOH fraction concentrations. Symbols with error bars represent means±SEM (n=6). The line represents the data fits to the Hill equation.
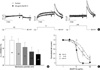
Fig. 6
Relative change in sustained HERG currents in response to H2O or BuOH fractions of L. erythrocarpa. (A, C) Original recording of currents under control conditions (Control) and in the presence of H2O (A) or BuOH (C) fraction of L. erythrocarpa (100 µg/mL each) during voltage steps to 0 mV. After having recorded the control measurement, the oocyte was clamped at -70 mV for 13 min during superfusion with the each fraction. (B, D) Relative current (Irel) obtained by dividing the H2O (C) or BuOH (D) fraction current by the control currents of the recording in A or C, respectively. Time 0 ms corresponds to the beginning of the depolarizing voltage step.

References
1. Sun BY, Chung YH. Monbographic study of the Lauracea in Korea. Kor J Plant Tax. 1988. 18:133–151.
2. Liu SY, Hisada S, Inagaki I. Terpenses of Lindera erythrocarpa. Phytochemistry. 1973. 12:233.
3. Liu SY, Hisada S, Inagaki I. Constituents of Lindera erythrocarpa. Phytochemistry. 1973. 12:472.
4. Oh HM, Choi SK, Lee JM, Lee SK, Kim HY, Han DC, Kim HM, Son KH, Kwon BM. Cyclopentenediones, inhibitors of farnesyl protein transferase and anti-tumor compounds, isolated from the fruit of Lindera erythrocarpa Makino. Bioorg Med Chem. 2005. 13:6182–6187.
5. Lee SM, Baek SH, Lee CH, Lee HB, Kho YH. Cytotoxicity of Lignans from Lindera erythrocarpa Makino. Nat Prod Sci. 2002. 8:100–102.
6. Sanguinetti MC, Jurkiewicz NK. Two components of cardiac delayed rectifier K+ current. Differential sensitivity to block by class III anti-arrhythmic agents. J Gen Physiol. 1990. 96:195–215.
7. Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996. 107:611–619.

8. Curran ME, Splawski I, Timothy KW, Vincent GM, Green ED, Keating MT. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell. 1995. 80:795–803.

9. Bianchi L, Wible B, Arcangeli A, Taglialatela M, Morra F, Castaldo P, Crociani O, Rosati B, Faravelli L, Olivotto M, Wanke E. Herg encodes a K+ current highly conserved in tumors of different histogenesis: a selective advantage for cancer cells? Cancer Res. 1998. 58:815–822.
10. Cherubini A, Taddei GL, Crociani O, Paglierani M, Buccoliero AM, Fontana L, Noci I, Borri P, Borrani E, Giachi M, Becchetti A, Rosati B, Wanke E, Olivotto M, Arcangeli A. HERG potassium channels are more frequently expressed in human endometrial cancer as compared to non-cancerous endometrium. Br J Cancer. 2000. 83:1722–1729.

11. Pardo LA, del Camino D, Sánchez A, Alves F, Brüggemann A, Beckh S, Stühmer W. Oncogenic potential of EAG K(+) channels. EMBO J. 1999. 18:5540–5547.

12. Suzuki T, Takimoto K. Selective expression of HERG and Kv2 channels influences proliferation of uterine cancer cells. Inter J Oncol. 2004. 25:153–159.

13. Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995. 81:299–307.
14. Kiehn J, Lacerda AE, Wible B, Brown AM. Molecular physiology and pharmacology of HERG. Single-channel currents and block by dofetilide. Circulation. 1996. 94:2572–2579.
15. Thomas D, Wendt-Nordahl G, Rockl K, Ficker E, Brown AM, Kiehn J. High-affinity blockade of human ether-a-go-go-related gene human cardiac potassium channels by the novel antiarrhythmic drug BRL-32872. J Pharmacol Exp Ther. 2001. 297:753–761.
16. Parihar AS, Coghlan MJ, Gopalakrishnan M, Shieh CC. Effects of intermediate-conductance Ca2+-activated K+ channel modulators on human prostate cancer cell proliferation. Eur J Pharmacol. 2003. 471:157–164.

17. Inglis V, Karpinski E, Benishin C. Gamma-dendrotoxin blocks large conductance Ca2+-activated K+ channels in neuroblastoma cells. Life Sci. 2003. 73:2291–2305.

18. Carignani C, Roncarati R, Rimini R, Terstappen GC. Pharmacological and molecular characterisation of SK3 channels in the TE671 human medulloblastoma cell line. Brain Res. 2002. 939:11–18.

19. Wu H, Wu K, Han Y, Shi Y, Yao L, Wang J, Fan D. Delayed rectifier K(+) channel regulated by cyclooxygenase-2 in human gastric cancer cell. Zhonghua Zhong Liu Za Zhi. 2002. 24:440–443.
20. Soliven B, Ma L, Bae H, Attali B, Sobko A, Iwase T. PDGF upregulates delayed rectifier via Src family kinases and sphingosine kinase in oligodendroglial progenitors. Am J Physiol Cell Physiol. 2003. 284:C85–C93.
21. Wang H, Zhang Y, Cao L, Han H, Wang J, Yang B, Nattel S, Wang Z. HERG K+ channel, a regulator of tumor cell apoptosis and proliferation. Cancer Res. 2002. 62:4843–4848.
22. Cone CD Jr, Tongier M Jr. Control of somatic cell mitosis by simulated changes in the transmembrane potential level. Oncology. 1971. 25:168–182.

23. Rim YS, Park YM, Park MS, Kim KY, Kim MJ, Choi YH. Screening of antioxidants and antimicrobial activity in native plants. Korean J Med Crop Sci. 2000. 8:342–350.
24. Suessbrich H, Schonherr R, Heinemann SH, Attali B, Lang F, Busch AE. The inhibitory effect of the antipsychotic drug haloperidol on HERG potassium channels expressed in Xenopus oocytes. Br J Pharmacol. 1997. 120:968–974.

25. Suessbrich H, Waldegger S, Lang F, Busch AE. Blockade of HERG channels expressed in Xenopus oocytes by the histamine receptor antagonists terfenadine and astemizole. FEBS Lett. 1996. 385:77–80.

26. Rampe D, Roy ML, Dennis A, Brown AM. A mechanism for the proarrhythmic effects of cisapride (Propulsid): high affinity blockade of the human cardiac potassium channel HERG. FEBS Lett. 1997. 417:28–32.

27. Green WN, Andersen OS. Surface charges and ion channel function. Annu Rev Physiol. 1991. 53:341–359.

28. Hille B. Ionic channels of excitable membranes. 1992. 2nd ed. Sunderland, Mass: Sinauer Associates Inc;459–461.
29. Wang N, Minatoguchi S, Arai M, Uno Y, Hashimoto K, Xue-Hai C, Fukuda K, Akao S, Takemura G, Fujiwara H. Lindera strychnifolia is protective against post-ischemic myocardial dysfunction through scavenging hydroxyl radicals and opening the mitochondrial KATP channels in isolated rat hearts. Am J Chin Med. 2004. 32:587–598.

30. Su MJ, Nieh YC, Huang HW, Chen CC. Dicentrine, an alpha-adrenoceptor antagonist with sodium and potassium channel blocking activities. Naunyn-Schmiedebergs Arch Pharmacol. 1994. 349:42–49.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download