This article has been
cited by other articles in ScienceCentral.
Abstract
The lengths of the surgical rectum and peritoneal reflection were important factors in treatment modality of rectal tumor. To evaluate the surgical length of rectum, we measured the length of the peritoneal reflections, sacral promontory and termination of the taenia coli from the anal verge by rigid sigmoidoscope in 23 male and 23 females during operation. The mean lengths of the sacral promontory were 16.5± 2.2 cm and 16.1±2.2 cm in the males and females, respectively. As for the peritoneal reflection, the results were anterior (8.8±2.2 cm, 8.1±1.7 cm), lateral (10.8±2.7 cm, 11.4±1.9 cm) and posterior (13.8±2.5 cm, 14.0±1.9 cm), respectively. There were no statistically significant differences between male and female. And only height had a correlation with the length of sacral promontory both in male and female (p=0.015 and p=0.018, respectively). For all the estimated lengths, the length of the sacral promontory had a correlation with the lengths of the anterior (p<0.001 and p=0.001) and posterior (p<0.001 and p<0.001) peritoneal reflections in males and females, respectively. We suggest that the intra-operative lengths of the rectum and peritoneal reflection will be useful information for treatment modality of rectal tumor clinically in Korean.
Keywords: Rectum, Sacral Promontory, Peritoneal Reflection
INTRODUCTION
The location of tumor in the rectum is an important factor that affects the choice of treatment modality. But there are various descriptions of the rectum.
Pathologically the rectum has mesorectum without taenia, epiploic appendices and haustra. The rectum is entirely extraperitoneal on its posterior aspect. The upper third of the rectum is covered anteriorly and laterally by the peritoneum. Finally, the lower third of the rectum is entirely extraperitoneal for the descriptive anatomy.
Surgically important marks of the rectum have been emphasized on the proximal limits of the rectum and the edges of the peritoneum because those limits were important standards for medical management of rectal tumor (
1). But the proximal limits of both the rectum and the peritoneal reflection are debatable. The rectosigmoid junction is considered to be at the S3 level by anatomists or at the sacral promontory by surgeons. There have also been some efforts to check the length of the peritoneal reflection as the proximal limit of the rectum (
1-
3). But in that study, the presented lengths of the rectum were checked in cadaver, so there was a difference for the rectum lengths of live human.
Because the treatment modality for a rectal neoplasm, such as preoperative chemoradiation or the methods of operation, is determined by the tumor's location from the anal verge, the preoperative practical lengths of the rectum for live human is an important factor for treatment decisions.
So in this study, we have evaluated and compared the length of the surgical rectum and peritoneal reflection in live human to provide information for treatment of rectal tumor.
MATERIALS AND METHODS
We prospectively collected data from 46 patients (23 males and 23 females) who underwent laparotomy for any reason from May 2006 to January 2007. The patients who had lesions that had the possibility of changing the pelvic anatomy (i.e., rectal cancer or a previous pelvic operation) were excluded from our study.
The collected data were the lengths of the anterior, lateral and posterior peritoneal reflections, the sacral promontory and the termination of the taenia coli from the anal verge, and these were all determined via a rigid sigmoidoscope; in addition, we determined the patients' age, height, weight and body mass index (BMI), the reason for laparotomy and the complications from the rigid sigmoidoscope.
Under general anesthesia, laparotomy was done with the patient in the lithotomy position. One investigator checked all examinations.
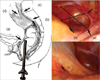
The conventional rigid sigmoidoscope that was used was 25 cm long and it had a diameter of 1.8 cm. All lengths were checked from the anal verge to the target point with performing hand palpation to remove all the air in the bowel (
Fig. 1).
The Shapiro-Wilk Test was used for normally distributed data. Statistical differences for the length of the peritoneal reflection and sacral promontory measurements between the genders were compared by using Student's t-test. The correlation analysis was performed with using Pearson's correlation method. Regression analysis was used for evaluating the relation equation. The level of confidence was defined as p<0.05.
RESULTS
The mean age of the enrolled 23 males and 23 female patients were 59.4 yr (range: 37.0-78.0 yr) and 55.9 yr (range: 23.0-77.0 yr), respectively. The patient demographics are shown in
Table 1. The mean lengths of the sacral promontory were 16.5±2.2 cm and 16.1±2.2 cm in the males and females, respectively. And the mean lengths of the taeniea coli were 18.6±2.4 cm and 17.6±3.3 cm. The mean lengths of the peritoneal reflection were anterior (8.8±2.2 cm, 8.1±1.7 cm), lateral (10.8±2.7 cm, 11.4±1.9 cm) and posterior (13.8±2.5 cm, 14.0±1.9 cm), respectively. There were no statistically significant differences in the lengths of the peritoneal reflection (anterior:
p=0.263, lateral:
p=0.368, posterior:
p=0.691), the sacral promontory (
p=0.528) and the termination of the taenia coli (
p=0.289) between the males and females (
Table 2). There was no postoperative morbidity due to rigid sigmoidoscope.
On the normality test (the Shapiro-Wilk test), the estimated value and clinical factors showed a normal probability distribution except for the length of lateral peritoneal reflection in the males and the females.
Correlation between the estimated values and clinical factors
For both the enrolled males and females, only height had a correlation with the length of the sacral promontory as a clinical factor (
p=0.015 and
p=0.018 for the males and females, respectively). For the estimated lengths, the length of the sacral promontory had a correlation with the lengths of the anterior and posterior peritoneal reflections (
Table 3,
4,
Fig. 2,
3).
By means of relationship between the length of peritoneal reflection and height, a relation equation was created that predicts the lengths of sacral promontory, in relation to the anal verge, from the patient's height according to the logistic regression analysis.
And because the length of peritoneal reflection had a correlation with sacral promontory and the length of sacral promontory had a correlation with patient's heights, the lengths of anterior and posterior peritoneal reflection would be prospected by the patient's height by the two logistic regression analyses. But the equations for peritoneal reflection did not have a statistical significance.
For males, the equations that predict the length of sacral promontory was
The length of sacral promontory (male, cm)=-10.0+0.2×height (cm) (p=0.01, coefficient of determination R2=0.26)
The length of anterior peritoneal reflection (male, cm)=-9.7+0.1×height (cm) (p=0.13, coefficient of determination R2=0.11)
The length of posterior peritoneal reflection (male, cm)=-12.9+0.2×height (cm) (p=0.06, coefficient of determination R2=0.18)
For females,
The length of sacral promontory (female, cm)=-5.1+0.1×height (cm) (p=0.01, coefficient of determination R2=0.27)
The length of anterior peritoneal reflection (female, cm)=-6.3+0.1×height (cm) (p=0.09, coefficient of determination R2=0.14)
The length of posterior peritoneal reflection (female, cm)=0.4+0.1×height (cm) (p=0.12, coefficient of determination R2=0.12)
DISCUSSION
Neoplasms of the rectum are common and their management requires detailed knowledge of the pelvic anatomy. With the evolution of surgical techniques, there are now various surgical methods for treating rectal tumor such as anterior resection, low anterior resection, abdominoperineal resection and especially transanal local excision or transanal endoscopic microsurgery (TEM).
TEM has been developed for treating early rectal cancer and benign neoplasm; it has been primarily used for local excision of selected low, middle, and upper rectal tumors via the anus. If the lesion is adequately identified in the rectum and located distal to the peritoneal reflection, then transanal local excision can be used. If the tumor is located above the peritoneal reflection, then the danger of injury to the bowel is increased with opening of the peritoneal reflection. Therefore, defining the length of rectum was important for consideration of transanal local excision.
As preoperative neoadjuvant chemoradiation has recently gained acceptance for the treatment of lower and mid rectal cancer, the exact localization of the primary rectal tumor is thought to be also very important for determining the choice of treatment modality. Some radiologists have reported that those lesions within 15 cm from the anal verge can be considered rectum, and so they are amenable to neoadjuvant treatment prior to surgery (
4,
5). Practically for clinicians, however, the upper boundary of the irradiated field is not the end of the cancer from the anal verge but the upper boarder of the sacral promontory as rectum between L5 and S1 from the anal verge (
6).
The clinical or anatomical definition of the rectum is of primary importance for the management for rectal tumor, but there have been various definitions regarding the upper boarder of the rectum, and these definitions have been a matter of debate for a long time.
The rectum is a linear organ in lower mammals and the word rectum is derived from the Latin word rectus, which means straight. In humans, however, the rectum is curved and follows the shape of the sacrum and coccyx (
7). There are also considerable differences in the terminology between anatomists and surgeons. Some authors have reported that the most useful landmark, both functionally and anatomically, for the transition from the sigmoid colon to the rectum, is the loss of the taenia coli, the appendices epiploicae and the surgical mesocolon at about the level of the third sacral vertebra at the rectosigmoid junction, where the superior rectal artery and a couple of the hypogastric nerves enter the pelvic cavity (
7-
13). Clinically, the proximal end of rectum is the position of the peritoneal reflection at the level of the sacral promontory; this is achieved by the recto-sacral fascia, which is at the last 12-15 cm from the anal verge, when this is measured using a rigid proctosigmoidoscope (
7,
12,
14). In our opinion, the level of the sacral promontory is most applicable to the definition of the upper boarder of the rectum when considering the upper boarder of the radiation field and the anatomical and clinical applicable definition for the rectum.
Actually, there have been few reports dealing with the relations among the levels of loss of the taenia coli and the positions of the peritoneal reflection and sacral promontory in live human. There is also a great individual variation in the gross anatomy of the pelvis and the lengths of the rectum according to age, gender, the nutritional status and constitutional factors. Several studies have investigated the length of the peri-rectal anatomy but these were based on cadaver measurements (
10,
15). In our study, we examined the length of the sacral promontory as the upper boarder of the rectum to the anal verge in live patients. The mean lengths of the sacral promontory were 16.5±2.2 cm and 16.1±2.2 cm in males and females, respectively, without a statistical difference.
As was stated above, transanal local excision of rectal tumor can endanger the intraabdominal organs if the procedure is done above the peritoneal reflection. So the upper border of the peritoneal reflection is important, but the position and length of the peritoneal reflection in humans are highly variable. By means of using the anatomic reference of the second rectal valve, which is the most consistently located valve and it correlates with the anterior peritoneal reflection, the anterior peritoneal reflection is approximately 8 and 6 cm from the anal verge in men and women, respectively. The posterior peritoneal reflection occurs between 12 and 15 cm in the male and female (
7).
Some authors have reported that the length of the peritoneal reflection is 5.5-12 cm anteriorly, 15 cm laterally and 20 cm posteriorly, which were determined by cadaveric dissections (
16,
17). But fixed cadaveric measurements of the lengths of the peritoneal reflection may not be applicable to live patients.
For clinical applications, there have been some studies onlocating the site of the peritoneal reflection in relation to rectal lesions in live humans. Gerdes et al. (
2) used transrectal ultrasound (TRUS) to demonstrate that TRUS is able to exactly determine the location of a rectal tumor with regard to the peritoneal reflection. Najarian et al. (
3) measured the length of the extraperitoneal rectum via proctoscopy in patients who were undergoing laparotomy. In that study, there were no differences between the males and females. For the males, weight and BMI had much stronger correlations than those for the females, and height was not predictive of the length of the peritoneal reflection. Some authors have reported that in obese women, and especially those who are short, the fat accumulation makes the meso-rectum thick and the peritoneum at the anterior and lateral walls hangs loosely into the pelvic cavity (
13).
In our study, the males' mean lengths of the anterior, lateral and posterior peritoneal reflections from the anal verge were 8.8±2.2 cm, 10.8±2.7 cm, and 13.8±2.5 cm, respectively. For the female, those values were 8.1±1.7 cm, 11.4±1.9 cm, and 14.0±1.9 cm, respectively. There was no statistical difference between the males and females. And the only statistical correlation between the examined lengths and clinical factors was for the length of the sacral promontory and height in both genders. The lengths of the anterior and posterior peritoneal reflection were also statistically correlated with the length of the sacral promontory. With a better understanding of the surgical anatomy of the rectum, the estimated results of surgical length will enable the surgeon to perform a more correct and reasonable procedure. As was mentioned above, even if the enrolled patients were any amount on subject, the statistical equations about the length of sacral promontory from our study also will be useful for clinical applications to determine the various treatment modalities of rectal tumors. And because of the small number of enrolled patients in our study, we expect more reliable equations for lengths of sacral promontory and peritoneal reflection by involving a larger series of patients.
In summary, there was no statistical difference in the estimated lengths between the males and females. On the statistical analysis, only height was correlated with the length of the sacral promontory both in males and females. The estimated lengths and presented equations will be helpful tools for determining the treatment of rectal tumor.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


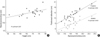



 XML Download
XML Download