Abstract
Although docetaxel monotherapy has shown clinical benefits for previously treated patients with advanced non-small cell lung cancer (NSCLC), the efficacy of salvage docetaxel chemotherapy for elderly patients or patients with poor performance status (PS) is controversial. Therefore, we conducted a phase II trial to evaluate the safety and efficacy of weekly low-dose docetaxel monotherapy in these patients. Forty NSCLC patients, who had been previously treated with one or more chemotherapy regimens, received docetaxel at a dose of 25 mg/m2 weekly on days 1, 8, and 15 of a 28-day cycle. All patients were ≥65 yr or had a PS of grade 2 in the cases of <65 yr. Weekly low-dose docetaxel was well-tolerated. Grade 3/4 non-hematologic toxicities were rare; fatigue in 3 patients (8%), anorexia in 3 patients (8%) and stomatitis in 2 patients (5%). Grade 3/4 neutropenia was noted in only one patient (3%). By intent-to-treat analysis, nine patients (23%) had partial responses and eleven patients (28%) demonstrated stable disease. The median progression-free survival and overall survival were 9.9 weeks and 37.7 weeks, respectively. Weekly low-dose docetaxel therapy provides a reasonable alternative for NSCLC salvage treatment in pretreated elderly patients or in patients with a reduced PS.
Lung cancer is the main cause of cancer-related death worldwide. In Korea, according to the statistics reported in 2005, lung cancer is the second and fifth most common cancer in men and women, respectively. Moreover, lung cancer is the most common cancer in elderly men (≥65 age group) (1). Non-small cell lung cancer (NSCLC) is found in >80% of all cases; most patients present with advanced or metastatic disease. These cases with advanced stages (IIIB or IV) cannot be cured with current therapies. Thus, life prolongation and symptom palliation are the main goals of treatment for these patients.
The standard first-line treatment for advanced NSCLC, in patients with good performance status (PS), consists of platinum-based combinations. This therapy has been shown to provide a survival advantage over supportive care alone (2). However, despite adequate first-line chemotherapy, the majority of patients will experience disease progression. In the setting of second-line treatment, docetaxel has demonstrated superiority for 1-yr survival and quality of life compared with ifosfamide, vinorelbine or supportive care alone (3, 4); at a recommended dose of 75 mg/m2 every 3 weeks.
Elderly patients or patients with poor PS have been historically excluded from large randomized clinical trials, and thus limited evidence-based data are available to guide the treatment of these patients. Single agent therapy remains a valid option for elderly patients with NSCLC (5); a large Italian study failed to demonstrate a benefit for combination therapy over single agent therapy (6). For patients with poor PS, data are more scant and few randomized trials comparing single-agent with combination chemotherapy have been reported in this subgroup. The recent American Society of Clinical Oncology (ASCO) guidelines recommended single agent therapy for the treatment of elderly and PS 2 patients (7).
During the initial introduction of docetaxel, it was routinely administered once every 3 weeks. However, although docetaxel was effective salvage treatment in patients previously treated with platinum-based chemotherapy, hematological toxicity was substantial with the 3-week treatment schedule; grade 3 or 4 neutropenia was observed in 54-67% of patients treated with a 75 mg/m2 dose of docetaxel (3, 4, 8). Even at a 60 mg/m2 dose, grade 3 or 4 neutropenia developed in more than 80% of patients in a Japanese trial (9). However, recent randomized clinical trials showed that docetaxel on a weekly schedule has a similar efficacy as the 3-weekly regimen and has less severe neutropenia (10-13). Patients with advanced age or poor PS are usually not candidates for the 3-weekly full-dose docetaxel monotherapy due to the severe toxicities. Therefore, treatment with weekly docetaxel provides an attractive treatment option for elderly or less fit patients with poor PS.
Based on the efficacy of salvage docetaxel chemotherapy in NSCLC and its relatively favorable toxicity profiles, when used on a weekly schedule, we conducted this phase II trial to test the safety and efficacy of weekly docetaxel monotherapy in previously treated elderly or less fit patients with poor PS. A weekly low-dose regimen of docetaxel (25 mg/m2/week) was chosen, due to the fragility of the study population.
To be eligible for this study, patients were required to have: histologically confirmed stage IIIB (malignant effusion) or IV NSCLC that progressed after one or more prior chemotherapeutic regimens. Patients who were ≥65 yr with an Eastern Cooperative Oncology Group (ECOG) PS of grade ≤2 were enrolled. In the cases where the patients were <65 yr, the ECOG PS should be grade 2. All patients were required to have at least one measurable disease (defined as a mass with demarcated dimensions by computed tomography [CT], routine chest radiography or by physical examination). In addition, adequate hematological counts were required (absolute neutrophil count [ANC] ≥1.5×109/L and platelet count ≥100×109/L) and laboratory results had to be within the following limits (serum bilirubin ≤1.25×upper normal limit [UNL], serum aspartate aminotransferase [AST] and alanine aminotransferase [ALT] ≤1.5×UNL, serum alkaline phosphatase ≤5.0×UNL, and serum creatinine ≤1.5 mg/dL). Patients treated for brain metastases by radiation were eligible if they were neurologically stable.
Patients were not eligible if they had a history of a prior or a concomitant malignancy, except for cases with treated non-melanoma skin cancer or in situ cervical cancer. Patients with pre-existing sensory or motor neurological symptoms of ≥grade 2 according to the National Cancer Institute Common Toxicity Criteria (NCI-CTC) were excluded as well as patients with active infections or other serious underlying medical conditions that might have impaired the ability of the patient to receive the planned protocol of treatment. All patients gave written informed consent before initiating treatment according to institutional regulations.
Before initiation of the chemotherapy, the patients were evaluated as follows: medical history, physical examination, determination of ECOG PS, complete blood cell counts, biochemical tests and tumor evaluation. A chest radiography and chest CT scan (including liver and adrenal glands) were required. A bone scan and brain imaging were performed only if clinically indicated.
Docetaxel 25 mg/m2 was administered weekly intravenously (i.v.) as a 1-hr infusion on an outpatient basis. Docetaxel was given for three consecutive weeks. This was followed by a treatment-free week. These cycles were repeated every 4 weeks for a maximum of 6 cycles. The premedication was as follows: one dose of 10 mg i.v. dexamethasone (30 min before chemotherapy) and two doses of 8 mg oral dexamethasone (12 hr and 24 hr after chemotherapy). All patients received adequate antiemetic therapy prior to the chemotherapy. Granulocyte colony-stimulating factor (G-CSF) was not routinely administered.
Dose modifications based on hematological toxicity were made as follows: at the start of each cycle, the ANC was required to be ≥1,500/µL and the platelet count ≥75,000/µL. If the ANC was <1,500/µL or the platelet count was <75,000/µL, the treatment was withheld that week, and the blood counts were reevaluated the following week. The treatment proceeded as scheduled if the blood cell count increased to an ANC ≥1,500/µL and a platelet count ≥75,000/µL. A 25% dose reduction was required if patients developed a grade 4 neutropenia, febrile neutropenia or a grade 4 thrombocytopenia. Dose adjustments were also made for patients with non-hematological toxicities. When a non-hematological toxicity of grade ≥3 developed, with the exception of alopecia or emesis, the dose of docetaxel was held until the toxicity resolved to less than grade 2, and then the docetaxel was given at 75% of the initial dose. If the non-hematological toxicity was grade 2, the dose of docetaxel was held until the toxicity resolved to a grade ≤1 and then the docetaxel was given at a dose of 25 mg/m2. The missed doses were not made up. Patients were withdrawn from the study when the delay of treatment was greater than one cycle, i.e. 4 weeks. The chemotherapy was discontinued for patients with progressive disease (PD) or unacceptable toxicity.
Physical examination, complete blood counts and biochemical tests were carried out before each cycle of therapy. Tumors were measured every 2 cycles (8 weeks) by imaging studies using the same procedures described for the pretreatment evaluation. The responses were assessed using the Response Evaluation Criteria in Solid Tumors (RECIST). A complete response (CR) was defined as the disappearance of all clinical evidence of tumor. A partial response (PR) was defined as a sustained ≥30% decrease in the sum of the longest diameter (LD) of target lesions, taking as reference the baseline sum LD. Confirmatory CT scans after 4 weeks in patients with CR or PR were not performed if patients' symptoms, physical examination or chest-x ray before the next chemotherapy cycle did not have the evidence of disease progression. PD was defined as at least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of new lesions. Stable disease (SD) was defined as a tumor response that did not meet the above CR, PR or PD criteria. Toxicities were evaluated using the NCI-CTC (version 3.0) before each treatment.
The primary study endpoint was the response rate to weekly low-dose docetaxel treatment. Progression-free survival (PFS) and overall survival (OS) were secondary endpoints. The PFS was calculated from the first day of chemotherapy to the date of disease progression, death from any cause or to the date of the last follow-up if none of the preceding events had occurred. OS was calculated from the first day of chemotherapy to the date of death or the last follow-up visit. Analyses of PFS and OS curves were performed using the Kaplan-Meier method.
This trial was designed to detect a response rate of 20% compared to a minimal, clinically meaningful response rate of 5%. An optimal Simon two-step design was used (14), with a power of 90% to accept the hypothesis and a 10% significance to reject the hypothesis. Twelve patients were initially recruited, with the intention that if no response was observed, the trial would have been discontinued. However, the trial would continue and 37 patients would be recruited to evaluate the response to weekly docetaxel chemotherapy, if at least one of the 12 patients showed an objective response.
From May 2004 to January 2007, 40 patients were enrolled at Seoul National University Bundang Hospital. The patient characteristics are shown in Table 1. There were 24 males and 16 females with a median age of 66 yr (range: 33-80 yr). Thirty patients (75%) had an ECOG PS of 2. Among 23 patients aged ≥65 yr, 13 patients had an ECOG PS of 2. Thirteen patients were aged 70 yr or older. All patients had measurable tumor lesions. Lung (68%), lymph nodes (65%), pleura (63%), bone (40%), and adrenal glands (20%) were the most common metastatic sites.
Twenty-nine patients (73%) received weekly low-dose docetaxel as second-line chemotherapy, eight patients (20%) as third-line therapy and three patients (8%) as fourth-line treatment. Most of the patients (≥90%) were previously treated with gemcitabine plus platinum combination chemotherapy as first-line therapy. Among 11 patients who received weekly docetaxel as ≥third-line chemotherapy, nine patients had been previously exposed to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors. As a whole, platinum, gemcitabine, paclitaxel, and EGFR inhibitors were previously employed in 39 (98%), 37 (93%), 9 (23%), and 9 patients (23%), respectively.
Among the 40 patients who received chemotherapy, 3 patients (8%) dropped out early before the response evaluation for the chemotherapy; two patients who had severe fatigue rejected further treatment and one patient discontinued the study treatment due to early death (respiratory failure caused by severe pneumonia).
There were 112 treatment cycles delivered, with a median of 2 cycles per patient (range: 1-6). Five patients (13%) received the planned 6 cycles of chemotherapy. The reasons for premature treatment cessation were disease progression (n=25), toxicity (n=6), concurrent disease (n=1), or the patient's decision to end treatment (n=3). The relative dose intensity (RDI) was calculated according to the method described by Hryniuk (15). The calculated mean dose intensity for docetaxel was 17.8 mg/m2/week and the RDI was 95%.
Of the 40 patients who received chemotherapy, three patients (8%) could not be evaluated for a response. By the intent-to-treat analysis, the overall response rate was 23% (95% confidence interval [CI]: 12-38%); nine of the 40 patients achieved a PR. Eleven patients (28%) had SD, and 17 patients (43%) had tumor progression. The disease control rate (CR+PR+SD) was thus 50%. Among the 13 patients who were ≥70 yr (ECOG PS 0-2), 3 patients (23%) had a PR. In addition, PR was observed in two patients (15%) out of the 13 patients who were ≥65 yr and had an ECOG PS of grade 2.
Among the 29 patients who received weekly docetaxel as a second-line therapy, five patients achieved a PR. Four of the 11 patients treated with weekly docetaxel as ≥third-line treatment had a PR. Of the 28 patients who had experienced clinical benefits (CR, PR or SD) from first-line chemotherapy, 8 patients (29%) achieved a PR after salvage weekly docetaxel chemotherapy. However, among the 12 patients who had shown PD after first-line chemotherapy, only one patient (8%) achieved a PR after salvage docetaxel treatment.
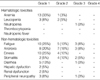
Toxicities were evaluated in all 40 patients and the results are summarized in Table 2. The most common hematological toxicity was anemia (35%). Of the grade 3 or 4 hematological toxicities, there was only one case (3%) of grade 3 neutropenia. No patient experienced a febrile neutropenia and no grade ≥3 anemia or thrombocytopenia was observed. Non-hematological toxicities consisted mainly of fatigue, anorexia, emesis, and stomatitis. Both fatigue and anorexia occurred in 18 patients (45%). However, severe fatigue or anorexia (grade ≥3) developed in three patients (8%). Nausea and vomiting occurred in 14 patients (35%) and no patient experienced severe emesis. Eight patients (20%) developed stomatitis, but only two (5%) patients suffered from severe stomatitis (grade ≥3). No patient experienced infusion-related hypersensitivity during the chemotherapy.
Six patients (15%) could not complete the planned schedule of chemotherapy because of related toxicities. Among these six patients, three patients could not be evaluated for response to chemotherapy. Three patients rejected receiving further treatment due to severe fatigue, and one patient could not receive further chemotherapy due to severe anorexia. One patient had a pulmonary thromboembolism during the chemotherapy and the docetaxel treatment was withdrawn. One patient discontinued the study treatment due to an early death (respiratory failure caused by severe pneumonia). Among the 13 patients who were ≥70 yr (ECOG PS 0-2), three patients (23%) could not complete the planned chemotherapy due to the adverse effects of the treatment. Out of the 13 patients who were ≥65 yr and had an ECOG PS of grade 2, the weekly docetaxel therapy was also permanently stopped in three patients (23%) because of chemotherapy-related toxicities.
The median follow-up duration of all patients was 25.9 weeks (range: 2.7-61.6 weeks) and the median follow-up duration of survivors was 35.3 weeks (range: 20.3-61.6 weeks). The median PFS of all patients (N=40) after the initiation of the weekly docetaxel chemotherapy was 9.9 weeks (95% CI, 7.0-12.8 weeks, Fig. 1). The median PFS of responders (N=9) was 24.4 weeks (95% CI, 22.0-26.8 weeks) and the median PFS of patients with SD (N=11) was 15.1 weeks (95% CI, 9.5-20.7 weeks), respectively. The median OS of all patents was 37.7 weeks (95% CI, 20.0-55.4 weeks, Fig. 2).
The results of our study support the safety and efficacy of weekly low-dose docetaxel (25 mg/m2) for the treatment of elderly NSCLC patients (≥65 yr) or patients of any age with poor PS who were previously treated with chemotherapy. The overall response rate was 23% with a median PFS and OS of 9.9 and 37.7 weeks, respectively. Compared with conventional 3-weekly or weekly higher-dose docetaxel regimens (10-13, 16-19), this weekly low-dose regimen showed similar efficacy as salvage therapy in NSCLC patients. Moreover, despite the fragility of the enrolled patients, the observed toxicities were generally well tolerated.
Hainsworth et al. reported that the maximum tolerated dose (MTD) of weekly docetaxel was 43 mg/m2/week in patients with advanced refractory tumors (20). Several clinical trials, using weekly docetaxel monotherapy, have been performed for NSCLC. However, these prior studies had different docetaxel doses (range, 25 to 40 mg/m2) and in addition, the sequence of weeks with and without treatment differed (10-13, 16-19, 21-24). Three randomized phase III trials that compared docetaxel administered weekly with every 3 weeks, in previously treated NSCLC patients, demonstrated that weekly docetaxel regimens had a similar response and survival rates and superior hematological toxicity profiles compared to the 3-weekly schedule (10-12). Based on the better toxicity profiles and no relevant difference in survival, weekly docetaxel chemotherapy appears to be a valid alternative to the conventional 3-week cycle for treatment of NSCLC patients as second-line chemotherapy (13). The weekly regimen may provide an attractive treatment option for elderly patients or those with poor PS who are usually not candidates for the full-dose 3-week docetaxel regimen.
The data available for weekly docetaxel chemotherapy targeting elderly patients or patients with poor PS is limited. A Minnie Pearl Cancer Research Network phase II trial performed in the elderly (≥65 yr old) or younger patients who were considered poor candidates for combination chemotherapy for NSCLC used a weekly docetaxel dose of 36 mg/m2 for 6 weeks as first-line treatment. An 18% response rate and moderate toxicities were reported (21). A French multicenter phase II study performed in elderly NSCLC patients (≥70 yr old), with a schedule of 30 mg/m2 for 6 consecutive weeks, followed by a 2-week treatment-free period as first-line therapy, showed acceptable toxicities and moderate activity (response rate 10%; median PFS and OS of 2.2 and 4.3 months, respectively) (22). In a phase II study performed in elderly (≥70 yr) or PS 2 patients with NSCLC in the United States, using docetaxel 30 mg/m2 on days 1, 8, and 15 every 4 weeks as first-line treatment, the response rate was 5.4% and the median PFS and OS were 2.3 and 6.7 months, respectively (23).
However, the adequate dosage for weekly docetaxel therapy in the elderly or poor PS patients is controversial. In one pharmacokinetic and toxicity study in Western elderly patients (≥65 yr old), weekly docetaxel administered at 35 mg/m2 for 3 weeks followed by a 1-week break caused a grade ≥3 toxicity in over half of the patients. As a result of these findings, the investigators recommended a starting dose of 26 mg/m2 for this weekly schedule and dose escalation with no toxicity (25). Asian elderly patients appear to have lower tolerance for weekly docetaxel than Western patients do. In a phase I trial performed in Japanese patients (≥70 yr old), docetaxel was administered on days 1, 8, and 15 for each 28-day cycle. The MTD was 30 mg/m2/week and a dose of 25 mg/m2/week was recommended for future clinical trials (26).
Our study is the first reported trial of weekly docetaxel therapy in previously treated elderly patients or PS 2 patients with advanced NSCLC as salvage treatment. Ninety-eight percent of patients were previously exposed to platinum-based chemotherapy and 28% of patients received weekly docetaxel therapy as ≥third-line treatment. Although patients included in this study were fragile patients, therapeutic outcomes of the weekly low-dose docetaxel in these patients were in line with those of other trials, where younger or fitter patients were enrolled and/or higher weekly doses of docetaxel were used (10-12, 16-19). However, our study has some limitations. First, the elderly and PS 2 patients may have different characteristics; it has become increasingly evident that trials evaluating treatments for patients with poor PS should be conducted separately from those evaluating elderly patients (23, 26). At the time when this study was conceived, both subsets were considered special populations, primarily due to a lack of evidence-based data from large randomized trials, and thus elderly patients with good PS and patients of any age with poor PS were enrolled simultaneously (21, 23). Second, elderly patients were included in this study on the basis of chronological age alone without the consideration of geriatric criteria taking into account the general condition of the patients, comorbidity, cognitive, or functional status. Recent reports suggest that the frailty of elderly patients for receiving chemotherapy should not be judged by age alone and selected fit-elderly patients can tolerate conventional chemotherapy as young patients (27). Despite these limitations, the results of our study confirm the safety and efficacy of weekly low-dose docetaxel in these patients.
In conclusion, weekly low-dose docetaxel monotherapy was well-tolerated as salvage chemotherapy for previously treated elderly or poor PS patients with NSCLC. This approach provides a reasonable alternative for pretreated elderly or less fit patients with NSCLC.
References
1. Shin HR, Won YJ, Jung KW, Kong HJ, Yim SH, Lee JK, Noh HI, Lee JK, Pisani P, Park JG, Ahn YO, Lee SY, Lee CW, Woo ZH, Lee TY, Choi JS, Yoo CI, Bae JM. Nationwide cancer incidence in Korea, 1999-2001; first result using the national cancer incidence database. Cancer Res Treat. 2005. 37:325–331.

2. Sorenson S, Glimelius B, Nygren P. A systematic overview of chemotherapy effects in non-small cell lung cancer. Acta Oncol. 2001. 40:327–339.
3. Shepherd FA, Dancey J, Ramlau R, Mattson K, Gralla R, O'Rourke M, Levitan N, Gressot L, Vincent M, Burkes R, Coughlin S, Kim Y, Berille J. Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol. 2000. 18:2095–2103.

4. Fossella FV, DeVore R, Kerr RN, Crawford J, Natale RR, Dunphy F, Kalman L, Miller V, Lee JS, Moore M, Gandara D, Karp D, Vokes E, Kris M, Kim Y, Gamza F, Hammershaimb L. Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. The TAX 320 Non-Small Cell Lung Cancer Study Group. J Clin Oncol. 2000. 18:2354–2362.
5. Gridelli C, Aapro M, Ardizzoni A, Balducci L, De Marinis F, Kelly K, Le Chevalier T, Manegold C, Perrone F, Rosell R, Shepherd F, De Petris L, Di Maio M, Langer C. Treatment of advanced non-small-cell lung cancer in the elderly: results of an international expert panel. J Clin Oncol. 2005. 23:3125–3137.

6. Gridelli C, Perrone F, Gallo C, Cigolari S, Rossi A, Piantedosi F, Barbera S, Ferrau F, Piazza E, Rosetti F, Clerici M, Bertetto O, Robbiati SF, Frontini L, Sacco C, Castiglione F, Favaretto A, Novello S, Migliorino MR, Gasparini G, Galetta D, Iaffaioli RV, Gebbia V. Chemotherapy for elderly patients with advanced non-small-cell lung cancer: the Multicenter Italian Lung Cancer in the Elderly Study (MILES) phase III randomized trial. J Natl Cancer Inst. 2003. 95:362–372.

7. Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT, Somerfield MR. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004. 22:330–353.

8. Shim BY, Kim CH, Song SH, Ahn MI, Hong EJ, Kim SW, Kim S, Jo MS, Cho DG, Cho KD, Yoo J, Kim HK. The safety and efficacy of second-line single docetaxel (75 mg/m2) therapy in advanced non-small cell lung cancer patients who were previously treated with platinum-based chemotherapy. Cancer Res Treat. 2005. 37:339–343.
9. Kudo S, Hino M, Fujita A, Igarashi T, Arita K, Niitani H, Taguchi T. Late phase II clinical study of RP56976 (docetaxel) in patients with non-small cell lung cancer. Gan To Kagaku Ryoho. 1994. 21:2617–2623.
10. Gridelli C, Gallo C, Di Maio M, Barletta E, Illiano A, Maione P, Salvagni S, Piantedosi FV, Palazzolo G, Caffo O, Ceribelli A, Falcone A, Mazzanti P, Brancaccio L, Capuano MA, Isa L, Barbera S, Perrone F. A randomised clinical trial of two docetaxel regimens (weekly vs 3 week) in the second-line treatment of non-small-cell lung cancer. The DISTAL 01 study. Br J Cancer. 2004. 91:1996–2004.

11. Schuette W, Nagel S, Blankenburg T, Lautenschlaeger C, Hans K, Schmidt EW, Dittrich I, Schweisfurth H, von Weikersthal LF, Raghavachar A, Reissig A, Serke M. Phase III study of second-line chemotherapy for advanced non-small-cell lung cancer with weekly compared with 3-weekly docetaxel. J Clin Oncol. 2005. 23:8389–8395.

12. Camps C, Massuti B, Jimenez A, Maestu I, Gomez RG, Isla D, Gonzalez JL, Almenar D, Blasco A, Rosell R, Carrato A, Vinolas N, Batista N, Giron CG, Galan A, Lopez M, Blanco R, Provencio M, Diz P, Felip E. Randomized phase III study of 3-weekly versus weekly docetaxel in pretreated advanced non-small-cell lung cancer: a Spanish Lung Cancer Group trial. Ann Oncol. 2006. 17:467–472.

13. Di Maio M, Perrone F, Chiodini P, Gallo C, Camps C, Schuette W, Quoix E, Tsai CM, Gridelli C. Individual patient data meta-analysis of docetaxel administered once every 3 weeks compared with once every week second-line treatment of advanced non-small-cell lung cancer. J Clin Oncol. 2007. 25:1377–1382.

14. Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989. 10:1–10.

15. Hryniuk WM, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990. 8:1935–1937.

16. Petrioli R, Pozzessere D, Messinese S, Sabatino M, Ceciarini F, Marsili S, Correale P, Fiaschi AI, Voltolini L, Gotti G, Francini G. Weekly low-dose docetaxel in advanced non-small cell lung cancer previously treated with two chemotherapy regimens. Lung Cancer. 2003. 39:85–89.

17. Ardizzoia A, Acquati M, Fagnani D, Giordano M, Visini M, Scanni A, Quattrone A, Fusco O, Vergani C, Casartelli C, Tagliabue P, Malugani F. Second line therapy with weekly low-dose docetaxel for pretreated non-small-cell lung carcinoma patients: a multicenter Italian phase II study. Lung. 2004. 182:1–8.

18. Gervais R, Ducolone A, Breton JL, Braun D, Lebeau B, Vaylet F, Debieuvre D, Pujol JL, Tredaniel J, Clouet P, Quoix E. Phase II randomised trial comparing docetaxel given every 3 weeks with weekly schedule as second-line therapy in patients with advanced non-small-cell lung cancer (NSCLC). Ann Oncol. 2005. 16:90–96.

19. Chen YM, Shih JF, Perng RP, Tsai CM, Whang-Peng J. A randomized trial of different docetaxel schedules in non-small cell lung cancer patients who failed previous platinum-based chemotherapy. Chest. 2006. 129:1031–1038.

20. Hainsworth JD, Burris HA 3rd, Erland JB, Thomas M, Greco FA. Phase I trial of docetaxel administered by weekly infusion in patients with advanced refractory cancer. J Clin Oncol. 1998. 16:2164–2168.

21. Hainsworth JD, Burris HA 3rd, Litchy S, Morrissey LH, Barton JH, Bradof JE, Greco FA. Weekly docetaxel in the treatment of elderly patients with advanced nonsmall cell lung carcinoma. A Minnie Pearl Cancer Research Network Phase II Trial. Cancer. 2000. 89:328–333.
22. Lecaer H, Barlesi F, Robinet G, Fournel P, Geriniere L, Bombaron P, Falchero L, Auliac JB, Crequit J, Chouaid C. An open multicenter phase II trial of weekly docetaxel for advanced-stage non-small-cell lung cancer in elderly patients with significant comorbidity and/or poor performance status: the GFPC 02-02b study. Lung Cancer. 2007. 57:72–78.

23. Lilenbaum R, Rubin M, Samuel J, Boros L, Chidiac T, Seigel L, Dowlati A, Graham P, Beaumont J, Du H. A randomized phase II trial of two schedules of docetaxel in elderly or poor performance status patients with advanced non-small cell lung cancer. J Thorac Oncol. 2007. 2:306–311.

24. Ko YH, Lee MA, Hong YS, Lee KS, Park HJ, Yoo IR, Kim YS, Kim YK, Jo KH, Wang YP, Lee KY, Kang JH. Docetaxel monotherapy as second-line treatment for pretreated advanced non-small cell lung cancer patients. Korean J Intern Med. 2007. 22:178–185.

25. Hurria A, Fleming MT, Baker SD, Kelly WK, Cutchall K, Panageas K, Caravelli J, Yeung H, Kris MG, Gomez J, Miller VA, D'Andrea G, Scher HI, Norton L, Hudis C. Pharmacokinetics and toxicity of weekly docetaxel in older patients. Clin Cancer Res. 2006. 12:6100–6105.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download