Abstract
To compare the stem niche in different culture conditions of limbal epithelial cells, the suspended human limbal epithelial cells (HLECs) were seeded on the 3T3-pretreated plates and the other suspended cells were plated on amniotic membranes (AMs) which were either cryo-preserved or freeze-dried. All were cultured for 10 to 12 days. Reverse transcription-polymerase chain reaction (RT-PCR) for ATP-binding casette, subfamily G, member 2 (ABCG2), p63, cytokeratin 12, and connexin 43 were performed in cultivated HLECs and their expression levels were compared. The mRNA expression of all markers examined showed no statistically significant differences between the cells on cryo-preserved and on freeze-dried AM. The expression of p63 and cytokeratin 12 in cultivated cells on AMs were significantly lower than those in 3T3-cocultured cells on RT-PCR and immunofluorescent staining. Cultivated HLECs on AMs showed reduced proliferation and differentiation while maintaining stem-property regardless of the preservative method of AM.
Once epithelial cells are extracted from the limbus and subsequently cultured in vitro, the stem-property of limbal epithelial cells may change, compared with the cells in vivo niche. Additionally, the stem-cell niche of the limbal epithelial cells can be affected by how they are cultivated in vitro; either with 3T3 co-culture or on amniotic membrane (AM). Especially, freeze-dried AM, which is recently used for ocular surface reconstruction (1), might affect the characteristics of stem-property of the limbal epithelial cells when it is used in the cell culture, compared with cryopreserved AM.
Phenotypic characterization of the putative limbal stem cells has been recently reported. Semiquantitative immunohistochemical staining revealed keratin 14/19, EGF receptor, integrin α9, p63, integrin beta 1, ATP-binding casette, subfamily G, member 2 (ABCG2), and nestin as possible positive markers and keratin 3/12, E-cadherin, involucrin, nestin, connexin 43, and Hoechst 33342 as possible negative markers for limbal stem cells (2-11).
Therefore, to understand the effect of in vitro niche on the stem-property of limbal epithelial cells, we evaluated the changes in expression of p63 and ABCG2 as the putative stem cell markers, and cytokeratin 3 and connexin 43 as differentiation markers when cultured on 3T3 feeder or AMs which is either freeze-dried or cryo-preserved.
In accordance with the tenets of the Declaration of Helsinki and with proper informed consent, 30 human corneoscleral rims were obtained within 8 hr after penetrating keratoplasty. Each tissue was treated with 0.05% trypsin and 0.01% ethylenediaminetetraacetic acid (EDTA) at 37℃ and then collected every 20 min for 4 times. NIH/3T3 (ATCC, Manassas, VA, U.S.A.) for a feeder layer was treated with 4 µg/mL of mitomycin C (Sigma, St. Louis, MO, U.S.A.) at 37℃ for 2 hr and was plated in 80% of confluence for co-culture with epithelial cells.
Harvested human limbal epithelial cells (HLECs) were seeded on the pre-treated 3T3 feeder containing plates with the number of 8.8×103 cells/cm2, or were plated on AMs, either cryo-preserved AM or freeze-dried AM (AmniSite-Cornea, Bioland Ltd, Cheonan, Korea), with the number of 8.7-26×104 cells/cm2 These cells were primarily cultured with the supplemented hormonal epithelial medium (SHEM) containing DMEM/F12 2:1, 10% fetal bovine serum (FBS), epithelial growth factor (EGF) 10 ng/mL, insulin 5 µg/mL, 0.1 nM choleratoxin, penicillin-streptomycin 50 IU/mL, 0.18 mM adenine, 4 mM glutamine, hydrocortisone 0.4 µg/mL, and 2 nM triiodothyronine for 10 to 12 days. Cryo-preserved AMs, which were prepared by the methods previously mentioned (12), or freeze-dried AMs were fixed in either Millicell-CM (Millipore Corp., Bedford, MA, U.S.A.) or tissue inserter (Bioland Ltd). Epithelial cells of AMs were scraped with a cell scraper (Costar, Columbia, MD, U.S.A.).
Total RNA was extracted from cultivated HLECs with 1 mL of Easy Blue® reagent (Intron Bio-Technologies, Seoul, Korea) following the manufacturer's protocol. RT-PCR (Peltier Thermal cycler PTC-200, MJ Research, Watertown, MA, U.S.A.) was performed using 1 µg total RNA in aliquots and PCR products were harvested when the amplification was in the mid-log phase after checking the kinetics of PCR reaction for each of the primer related genes. PCR was carried out at 95℃ for 5 min then 35 cycles of 95℃ for 60 sec, 60℃ for 60 sec and 72℃ for 60 sec, ant finally at 72℃ for 60 sec. The primers for human ABCG2, p63, connexin 43, cytokeratin12, and GAPDH were described in Table 1. All products were separated by 2% agarose gel electrophoresis and visualized with 0.05 mg/mL ethidium bromide. Semi-quantitative analysis was done using the ratio of ABCG2, p63, cytokeratin 12, and connexin 43 to GAPDH measured by image analyzer (Vilber lourmat, Marne-la-Vallee, France) and densitometry (Tina 2.0, Raytest, Straubenhardt, Germany). They were measured more than triplicates.
Immunocytochemistry for the cultivated HLECs on cryopreserved AM and 3T3 feeder layer was done after fixation with cold methanol (for nuclear protein staining) at 4℃ for 10 min or 10% neutral buffered formalin (A5472, Sigma-Aldrich, St. Louis, MO, U.S.A.) at 4℃ overnight (for staining of cell membrane protein and cytoplamic protein). After blocking with 5% normal goat serum in phosphate buffered solution (PBS) for 30 min, primary monoclonal antibodies against nuclear p63 (1:100) and cytokeratin 12 (1:100) were applied and incubated overnight at 4℃. Secondary antibodies were then applied, followed by counterstaining with Hoechst 33342 DNA binding dye.
Statistical analysis was performed using Sigmaster ver 1.0: SPSS software (Chicago, IL, U.S.A.). A non-parametric Mann-Whitney U test was used to evaluate the level of significance of differences for semi-quantitative comparison of RT-PCR results in each group. To evaluate the differences in mRNA expression between the amniotic membrane groups and 3T3 feeder group, we averaged the results of RT-PCR in the cryopreserved and freeze-dried AM groups and then compared it with those in the 3T3 feeder group. Significance was set at p<0.05.
HLECs seemed to slowly grow on AMs (Fig. 1A, B), compared with those cocultured with 3T3 feeders (Fig. 1C). The relative expression of mRNA of p63 and cytokeratin 12 of HLECs cultured on AM were significantly lower (p=0.028, 0.004) than those of 3T3-cocultured cells (Fig. 1D, F). The mRNA expression of all markers examined showed no statistically significant differences between the cells on cryopreserved and on freeze-dried AM (Fig. 1E).
We investigated the effect of different culture conditions on the stem-property of limbal epithelial cells. Recently, the phenotypic expression of the cultivated epithelial cells on AM had been thoroughly investigated (13-21). A lot of reports showed that the AM provided the stem-niche-like environment (15-21). In fact, the cells on AM tend to grow slowly compared with those cocultured with 3T3 feeder layers, suggesting AM may have an effect on the differentiation of the stem cells. Our results showed that the cells on AM expressed lower level of p63 and cytokeratin 12, compared with those cultivated with feeder layer, and it suggests the stem nichelike property of AM which provide less proliferative and less differentiating condition. The effects of AM on the HLECs were not different in the expression of ABCG2, p63, cytokeratin 12, and connexin 43 between cryopreserved and freezedried AMs. The composition of amniotic basement membrane more closely resembles the conjunctival basement membrane than the corneal basement membrane (22). This property of AM might be also involved in the stem-niche for the limbal stem cells. In fact, fibronectin and laminin were reported to be apparent in both cryo-preserved AM and freeze-dried AM even though the amount of type III collagen was less in freeze-dried AMs (23). Therefore, extracellular matrix of AM related to basement membrane seems to be less influenced by the preservative method, in contrast to the epithelium dose (23).
The proliferation rates of the cells in both cryo-preserved AM and freeze-dried AM are slower than that in the feeder alone. It took about 3 weeks for the cells in both cryo-preserved and freeze-dried AMs to reach upto 80% confluency, while it took less than 2 weeks for the cells with feeder alone. The stem property of the cells may be time-dependent in vitro microenvironment. Therefore, more numbers of the cells were seeded on AMs with the number of 8.7-26×104 cells/cm2 compared with that in feeder-cocultured cells. Cell density may also affect the proliferation rate in vitro culture. Low cell densities in vitro culture of neural precursor cells are reported to be associated with higher proliferation (24). However, considering initial differences of proliferation rate, this comparison is reasonable although the density-related effect is not totally excluded.
In conclusion, cultivation on the AM tend to reduce proliferation and differentiation potential maintaining stemniche regardless of the preservative method of AM (cryo-pre served or freeze-dried). It can be applicable for the further cell transplantation without the need of any xeno-sources like 3T3 feeders to keep the stem-properties of the niche using AM.
Figures and Tables
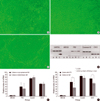 | Fig. 1Cultivated limbal epithelial cells on amniotic membranes (AMs) for 11 days. The limbal epithelial cells on cryopreserved AM (A) or freeze-dried AM (B) were slowly growing, compared with those with 3T3 feeders (C). Original magnification ×200. (D) RT-PCR results in limbal epithelial cells cultivated on cryopreserved (lane A) and freeze-dried amniotic membrane (lane B) or with 3T3 feeders (lane C). (E) The expression of all of them did not show any differences in cultivated epithelial cells between on cryo-preserved amniotic membrane and freeze-dried amniotic membrane. (F) The relative expression of mRNA for P63 and cytokeratin 12 was significantly lower in epithelial cells cultivated with amniotic membrane including both cryo-preserved and dried-freeze AM (p=0.028 for p63, 0.004 for cytokeratin 12, Mann Whitney U test) than that of 3T3-cocultured cells.
Conx 43, connexin 43; CK 12, cytokeratin 12. Arrow means the location of band for ABCG2 (110 bp).
|
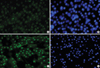 | Fig. 2Immunocytochemistry for p63 in cultivated limbal cells on the cryopreserved amniotic membrane (A) and 3T3 feeder payer (C). Low expression of p63 was noted in the cells cultured on AM. (B, D) Hoechst counterstaining. |
References
1. Nakamura T, Yoshitani M, Rigby H, Fullwood NJ, Ito W, Inatomi T, Sotozono C, Nakamura T, Shimizu Y, Kinoshita S. Sterilized, freeze-dried AM: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004. 45:93–99.
2. Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986. 103:49–62.

4. Chung EH, DeGregorio PG, Wasson M, Zieske JD. Epithelial regeneration after limbus-to-limbus debridement. Expression of alpha-enolase in stem and transient amplifying cells. Invest Ophthalmol Vis Sci. 1995. 36:1336–1343.
5. Pajoohesh-Ganji A, Ghosh SP, Stepp MA. Regional distribution of alpha9beta1 integrin within the limbus of the mouse ocular surface. Dev Dyn. 2004. 230:518–528.
6. Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. P63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2004. 98:3156–3161.

7. Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005. 118:1715–1724.

8. Seigel GM, Sun W, Salvi R, Campbell LM, Sullivan S, Reidy JJ. Human corneal stem cells display functional neuronal properties. Mol Vis. 2003. 9:159–163.
9. Zhao X, Das AV, Thoreson WB, James J, Wattnem TE, Rodriguez-Sierra J, Ahmad I. Adult corneal limbal epithelium: a model for studying neural potential of non-neural stem cells/progenitors. Dev Biol. 2002. 250:317–331.

10. Chen Z, de Paiva CS, Luo L, Kretzer FL, Pflugfelder SC, Li DQ. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004. 22:355–366.

11. Watanabe K, Nishida K, Yamato M, Umemoto T, Sumide T, Yamamoto K, Maeda N, Watanabe H, Okano T, Tano Y. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004. 565:6–10.

12. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995. 14:473–484.

13. Zhang X, Sun H, Tang X, Ji J, Li X, Sun J, Ma Z, Yuan J, Han ZC. Comparison of cell-suspension and explant culture of rabbit limbal epithelial cells. Exp Eye Res. 2005. 80:227–233.

14. Harkin DG, Barnard Z, Gillies P, Ainscough SL, Apel AJ. Analysis of p63 and cytokeratin expression in a cultivated limbal autograft used in the treatment of limbal stem cell deficiency. Br J Ophthalmol. 2004. 88:1154–1158.

15. Du Y, Chen J, Funderburgh JL, Zhu X, Li L. Functional reconstruction of rabbit corneal epithelium by human limbal cells cultured on amniotic membrane. Mol Vis. 2003. 9:635–643.
16. Grueterich M, Espana EM, Tseng SC. Ex vivo expansion of limbal epithelial stem cells: amniotic membrane serving as a stem cell niche. Surv Ophthalmol. 2003. 48:631–646.

17. Wang DY, Hsueh YJ, Yang VC, Chen JK. Propagation and phenotypic preservation of rabbit limbal epithelial cells on amniotic membrane. Invest Ophthalmol Vis Sci. 2003. 44:4698–4704.

18. Grueterich M, Espana EM, Tseng SC. Modulation of keratin and connexin expression in limbal epithelium expanded on denuded amniotic membrane with and without a 3T3 fibroblast feeder layer. Invest Ophthalmol Vis Sci. 2003. 44:4230–4236.

19. Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Expression of Delta Np63 in response to phorbol ester in human limbal epithelial cells expanded on intact human amniotic membrane. Invest Ophthalmol Vis Sci. 2003. 44:2959–2965.
20. Ban Y, Cooper LJ, Fullwood NJ, Nakamura T, Tsuzuki M, Koizumi N, Dota A, Mochida C, Kinoshita S. Comparison of ultrastructure, tight junction-related protein expression and barrier function of human corneal epithelial cells cultivated on amniotic membrane with and without air-lifting. Exp Eye Res. 2003. 76:735–743.

21. Hernandez Galindo EE, Theiss C, Steuhl KP, Meller D. Gap junctional communication in microinjected human limbal and peripheral corneal epithelial cells cultured on intact amniotic membrane. Exp Eye Res. 2003. 76:303–314.

22. Fukuda K, Chikama T, Nakamura M, Nishida T. Differential distribution of subchains of the basement membrane components type IV collagen and laminin among the amniotic membrane, cornea, and conjunctiva. Cornea. 1999. 18:73–79.

23. von Versen-Hoynck F, Syring C, Bachmann S, Moller DE. The influence of different preservation and sterilisation steps on the histological properties of amnion allografts--light and scanning electron microscopic studies. Cell Tissue Bank. 2004. 5:45–56.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download