Abstract
Angiogenin, a potent inducer of angiogenesis, is expressed in human endometrium. This study was performed to compare the expression of angiogenin mRNA level in the eutopic endometrium from women with and without endometriosis. Thirty-two women with advanced stage endometriosis and 29 control women were recruited. Following isolation of total RNA from endometrial tissue and reverse transcription, cDNA samples were amplified by real time polymerase chain reaction to quantify the expression of angiogenin genes. In selected patients, immunohistochemical staining was utilized to localize the area of angiogenin expression. Angiogenin mRNA level was significantly lower in the endometriosis group than in the control group during the secretory phase, especially the mid-secretory phase, and the decline was observed mainly in the women who presented with infertility. Within the endometriosis group, angiogenin mRNA levels did not differ between the proliferative and secretory phases, but, in the control group, the level in the secretory phase was higher than that during the proliferative phase. Immunohistochemistry showed that the glandular epithelial cell layer was decorated positively in both groups. These findings suggest that the relative deficiency of angiogenin expression in the secretory endometrium could impair implantation in women with advanced stage endometriosis.
The mechanisms by which endometriosis impairs fertility, as well as the etiologic factors responsible for its histogenesis and progression, remain poorly understood. There is growing evidence that the eutopic endometrium of women with endometriosis is different from that of women without endometriosis. Some of these differences may be related to failure of implantation, while others may contribute to the establishment and growth of endometriotic lesions (1-4).
Angiogenin is a heparin-binding 14.1-kDa single chain polypeptide that has been shown to have a higher angiogenic activity than other angiogenic factors (5). Angiogenin is expressed in human endometrium, with enhanced expression during the secretory phase, and in decidual tissues, raising the possibility that angiogenin may play a role in establishing pregnancy (6). Based upon the specific enhancement of angiogenin expression around the implantation window period along with its strong angiogenic activity, it is necessary to investigate whether the expression of angiogenin in the eutopic endometrium is different between women with and without endometriosis. This study was designed to compare the level of angiogenin expression in the eutopic endometrium from women with and without endometriosis by utilizing real-time polymerase chain reaction (PCR).
Endometrial tissues were obtained from a total of 61 patients who had undergone gynecological operations in their reproductive ages. Thirty-two patients had laparoscopic and histological evidence of advanced stage endometriosis, while 29 patients with proven fertility without the disease served as controls. The number of 30 patients for each group was initially chosen to detect a difference of 4.0 in the secretory phase between the two groups, assuming that a standard deviation is 4.0 and about half of the subjects are in the secretory phase, with a power of 0.8 for p<0.05. In the endometriosis group, the extent of disease was staged according to the American Society for Reproductive Medicine (1997) and we divided the patients into 2 subgroups; those who presented with infertility and those who presented with problems other than infertility. Endometrial samples were obtained either by using a curette after pelviscopy or by opening the uterine cavity immediately after hysterectomy. The review board for human research of Seoul National University Hospital approved this project, and written informed consent for use of the endometrium was obtained from each woman. All sections were dated by a gynecological pathologist according to the histological criteria of Noyes (7) and were classified as early proliferative (days 4-7), mid proliferative (days 8-10), late proliferative (days 11-14), early secretory (days 15-18), mid secretory (days 19-22) or late secretory (days 23-28) phases.
All patients in the study had regular menstrual cycles and had not received any hormonal therapy during the previous 6 months. The clinical characteristics, the menstrual phases of each group, and the stages of endometriosis are summarized in Table 1. Patients with a previous history of infertility and histological or sonographic evidence of adenomyosis or submucous myoma, which might be associated with infertility, were excluded from the control group. The indications for surgery amongst the control group were benign ovarian cyst (n=14), carcinoma in situ of the uterine cervix (n=10), and uterine intramural leiomyoma (n=5). There were no significant differences in age between the two groups, but the average number of deliveries was significantly lower in the endometriosis group (p<0.05).
Endometrial tissues used for mRNA extraction were snapfrozen in liquid nitrogen and stored at -70℃ until analyzed. The tissues for immunohistochemical staining were immersed in OCT compound (Tissue Tek, Elkhart, IN, U.S.A.) and stored at -70℃ until analyzed. Total RNA was isolated using Trizol reagent (Life Technologies Inc, Rockville, MD, U.S.A.) according to the manufacturer's protocol and stored at -70℃. RT was performed using the Promega Reverse Transcription System (Promega, Madison, WI, U.S.A.). Real-time PCR was used for relative gene expression using an ABI Prism 7700 Sequence Detection System Thermal Cycler (Applied Biosystems, Foster City, CA, U.S.A.). Primers and probes were designed using Primer Express 1.5 Software and synthesized commercially (Applied Biosystems). The primer and probe sequences for angiogenin are shown in Table 2, and commercially synthesized primer and probe were used for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Human GAPDH Endogenous Control, Applied Biosystems).
The optimal concentrations of all primers and probes were determined according to the optimization protocol provided by Applied Biosystems. A stock placental cDNA pool was prepared as a standard and used for all real-time assays. PCR reaction mixes for each standard and samples were prepared in separate tubes, using TaqMan Universal PCR master mix, primers, probe and cDNA. All samples were assayed in triplicate, and 25 µL aliquots were transferred to wells of a MicroAmp optical 96-well reaction plate (Applied Biosystems). The thermocycler parameters were 50℃ for 2 min and 95℃ for 10 min, followed by 40 cycles of 95℃ for 15 sec and 60℃ for 1 min. A GAPDH pre-designed assay from Applied Biosystems was used to measure the expression of the housekeeping gene GAPDH in each sample using the same standard preparation. Angiogenin gene expression was normalized with GAPDH gene expression in each sample and the ratio between angiogenin and GAPDH was expressed as angiogenin mRNA level in all samples.
We selected 2 endometrial samples in each menstrual phase for the endometriosis and control group. Immunohistochemical staining was performed as previously described by Koga et al. (6). Briefly, 6 µm cryostat sections cut from frozen tissues were mounted on poly-L-lysine-treated slides, fixed in cold acetone at -20℃ for 10 min, and treated with 3% hydrogen peroxide for 10 min to eliminate endogenous peroxidases. The sections were incubated with mouse monoclonal antibody to human angiogenin (1:25; Biogenesis, Poole, U.K.) overnight at 4℃. Control slides were incubated with nonimmune mouse IgG, at a concentration adjusted to that of the primary antibody. The sections were incubated with biotinylated horse antimouse IgG, followed by avidin peroxidase and diaminobenzidine, using the Vectastain Elite ABC kit (Vector Laboratories, Inc., Burlingame, CA, U.S.A.). All sections were counterstained with hematoxylin. As the primary purpose of immunohistochemical staining was to localize the area of angiogenin expression in the control and endometriosis group, we did not perform semiquantitative comparison between the two groups. Assessments of immunostaining were based on agreement among three independent observers who were blinded as to which sections were from patients with endometriosis and which were from control patients.
Comparisons of age, number of deliveries, and angiogenin mRNA level between two groups were performed using the Mann-Whitney U test for nonparametric data. Angiogenin mRNA levels within the endometriosis or control group were compared among the 5 or 6 different menstrual phases using the Kruskal-Wallis test, followed by the Mann-Whitney U test with Bonferonni correction, if significant. Angiogenin mRNA levels within the control group were compared among the 3 different subgroups according to the diagnosis using the Kruskal-Wallis test. Results with p<0.05 were considered significant.
The overall mean levels of angiogenin mRNA across all phases were not significantly different between the endometriosis and the control groups (0.80±0.19 vs. 1.43±0.40, mean±SEM). While there was no significant difference in the proliferative phase (0.92±0.44 vs. 0.96±0.28), the mean level of angiogenin mRNA was significantly lower during the secretory phase in the endometriosis group compared with the control group (0.72±0.14 vs. 2.21±0.92, p<0.05). Within the endometriosis group, the mean level of angiogenin mRNA did not differ between the proliferative and secretory phase (0.92±0.44 vs. 0.72±0.14), but, in the control group, the mean level of angiogenin mRNA in the secretory phase was significantly higher compared with that in the proliferative phase (0.96±0.28 vs. 2.21±0.92, p<0.05) (Fig. 1). The mean levels of angiogenin mRNA expression in the early, mid, and late proliferative and late secretory phases were not different in the endometriosis group compared with the respective phases in the control group, but, in the mid-secretory phase, the mean level was significantly lower in the endometriosis group than in the control group (0.55±0.16 vs. 1.69±0.45, p<0.05). The levels of angiogenin mRNA were not different among the 6 different phases within the endometriosis group and the 5 different phases within the control group (Fig. 1).
When the endometriosis group is divided into 2 subgroups, the statistically significant decline was observed only in the women who presented with infertility compared with the control group during the secretory and mid-secretory phases (0.64±0.19 vs. 2.21±0.92, p<0.05; 0.40±0.15 vs. 1.69 ±0.45, p<0.05, respectively) (Fig. 2). However, there were no diffefences in mean levels of angiogenin mRNA expression between the two groups.
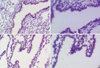
Immunohistochemical staining for angiogenin in the endometrium showed that the glandular epithelial cell layer and the stroma were decorated positively in both groups and that the localization of positive staining was not different between the two groups (Fig. 3).
Endometrial changes related to angiogenesis are associated with ovulation and serve to prepare a receptive nidation site for the embryo (8). Expression of vascular endothelial growth factor (VEGF) is increased in the secretory phase endometrium due to the effect of ovarian steroid hormones (9-11). Progesterone has been shown to increase expression of the endometrial VEGF189 isoform during the secretory phase of the menstrual cycle, and native VEGF189 has been observed to increase capillary permeability, which is necessary for implantation and possibly for the maintenance of pregnancy (12).
The findings of the present study suggest that the normal increase in angiogenin expression failed to occur during the implantation window period in the eutopic endometrium from women with advanced stage endometriosis. It is possible that a relative deficiency of angiogenin expression in patients with advanced stage endometriosis leads to a defect in the angiogenic process that is essential for successful implantation. At present, however, it is unclear whether the relative deficiency of angiogenin expression in the secretory phase is inherent to the eutopic endometrium itself or the secondary consequence of other factors associated with ovarian endometrioma. In addition, it has to be clarified whether angiogenin itself plays a critical role in the implantation window period or the expression of angiogenin in endometrial tissue is an epiphenomenon accompanying another critical event in the implantation window period. Further studies utilizing in vitro cell culture or animal models for analyzing the expression level of angiogenin and other established markers for implantation could give a correct answer to this issue.
Our results are in disagreement with recent reports (13, 14) demonstrating significant increases of angiogenin in the serum and peritoneal fluid in patients with endometriosis. Although it is very difficult to explain about the disagreement, it is possible that the expression of angiogenin in the eutopic endometrium is not regulated by the factors modulating the levels of circulating angiogenin. Alternatively, the elevated angiogenin levels in the peritoneal fluid of women with endometriosis may result from the inflammation processes associated with endometriosis. Despite lack of data, it may also be speculated that an increased level of angiogenin in the circulating blood or peritoneal fluid promote the establishment of endometriotic lesions in the pelvic cavity, and once endometriotic lesions have been established and progressed to advanced stage, unknown adverse events, such as impairment of luteal steroidogenesis or release of local inflammatory mediators inhibiting response to progesterone, reduce the expression of angiogenin in the eutopic endometrium.
Although the findings of the present study are novel, they may have several weaknesses. First, in the present study, immunohistochemistry was performed only in selected patients due to limited numbers of samples prepared, which made it impossible to evaluate intensity differences between the two groups with statistical comparisons. Second, we simply divided the endometriosis group into 2 subgroups only according to the chief complaints; women who presented with infertility and those who presented with problems other than infertility. One may argue that this comparison is meaningless since most women who presented with problems other than infertility can be proven to be infertile after long term follow-up or extensive work-up. Being well aware of the argument, we compared the level of angiogenin expression to evaluate whether there is a subtle difference between the two groups since some patients with endometriotic cyst can conceive easily without any treatment. Further, well-defined studies recruiting adequate number of patients with full information about fertility status can give a correct answer to these issues.
We designed this study as testing our hypothesis only in patients with stage III/IV endometriosis, as it was very difficult to recruit adequate number of histologically confirmed patients with stage I/II endometriosis. It is possible that the expression of angiogenin in the eutopic endometrium might be quite different between the patients with stage I/II and III/IV endometriosis, considering that immune status, role of growth factors, and pathogeneses are notably different for stage I/II and III/IV endometriosis (15-17).
Despite these limits, we have found that the expression of angiogenin is decreased in the eutopic endometrium from women with advanced stage endometriosis during the normal window of implantation. Although further studies are required to elucidate the relevance of angiogenin to the process of implantation, these findings suggest that the relative deficiency of angiogenin expression could be one of the critical factors involved in the mechanism by which endometriosis impairs fertility.
Figures and Tables
Fig. 1
Relative expression of angiogenin in the eutopic endometrium of women with and without endometriosis during menstrual phases.
*, Significant difference between proliferative and secretory phases (p<0.05); †,‡, Significant difference between patients and controls (p<0.05).
All data are expressed as mean±SEM.
WP, whole menstrual phase; P, proliferative phase; S, secretory phase; EP, early proliferative phase; MP, mid-proliferative phase; LP, late proliferative phase; ES, early secretory phase; MS, mid-secretory phase; LS, late secretory phase.
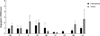
Fig. 2
Relative expression of angiogenin in the eutopic endometrium of women with and without endometriosis during the mid and late secretory phases.
*,†, Significant difference between controls and patients with endometriosis who presented with infertility (p<0.05).

Fig. 3
Immunohistochemical stains for angiogenin in the endometrium of the mid- secretory phase. Glandular epithelial cell layer is decorated positively (arrows) in both groups and the localization of positive staining was not different between the two groups.
(A) Control (Magnification, ×200), (B) Control (Magnification, ×400), (C) Endometriosis (Magnification, ×200), (D) Endometriosis (Magnification, ×400).
References
1. Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, Strom BL. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab. 1994. 79:643–649.

2. Healy DL, Rogers PA, Hii L, Wingfield M. Angiogenesis: a new theory for endometriosis. Hum Reprod Update. 1998. 4:736–740.
3. Giudice LC, Telles TL, Lobo S, Kao L. The molecular basis for implantation failure in endometriosis: on the road to discovery. Ann N Y Acad Sci. 2002. 955:252–264.
4. Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, Lessey BA, Giudice LC. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003. 144:2870–2881.

6. Koga K, Osuga Y, Tsutsumi O, Yano T, Yoshino O, Takai Y, Matsumi H, Hiroi H, Kugu K, Momoeda M, Fujiwara T, Taketani Y. Demonstration of angiogenin in human endometrium and its enhanced expression in endometrial tissues in the secretory phase and the decidua. J Clin Endocrinol Metab. 2001. 86:5609–5614.

7. Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Am J Obstet Gynecol. 1975. 122:262–263.

8. Sherer DM, Abulafia O. Angiogenesis during implantation, and placental and early embryonic development. Placenta. 2001. 22:1–13.

9. Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, Jaffe RB, Taylor RN. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996. 81:3112–3118.

10. Das SK, Chakraborty I, Wang J, Dey SK, Hoffman LH. Expression of vascular endothelial growth factor (VEGF) and VEGF-receptor messenger ribonucleic acids in the peri-implantation rabbit uterus. Biol Reprod. 1997. 56:1390–1399.
11. Lebovic DI, Shifren JL, Ryan IP, Mueller MD, Korn AP, Darney PD, Taylor RN. Ovarian steroid and cytokine modulation of human endometrial angiogenesis. Hum Reprod. 2000. 15S3:67–77.

12. Ancelin M, Buteau-Lozano H, Meduri G, Osborne-Pellegrin M, Sordello S, Plouet J, Perrot-Applanat M. A dynamic shift of VEGF isoforms with a transient and selective progesterone-induced expression of VEGF189 regulates angiogenesis and vascular permeability in human uterus. Proc Natl Acad Sci USA. 2002. 99:6023–6028.

13. Steff AM, Gagne D, Page M, Rioux A, Hugo P, Gosselin D. Serum concentrations of insulin-like growth factor-1, soluble tumor necrosis factor receptor-1 and angiogenin in endometriosis patients. Am J Reprod Immunol. 2004. 51:166–173.

14. Suzumori N, Zhao XX, Suzumori K. Elevated angiogenin levels in the peritoneal fluid of women with endometriosis correlate with the extent of the disorder. Fertil Steril. 2004. 82:93–96.

15. Arici A, Tazuke SI, Attar E, Kliman HJ, Olive DL. Interleukin-8 concentration in peritoneal fluid of patients with endometriosis and modulation of interleukin-8 expression in human mesothelial cells. Mol Hum Reprod. 1996. 2:40–45.




 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download