Abstract
A patient with renal cell carcinoma (RCC) developed synchronous bone metastasis with metachronous relapses to the bone and renal fossa. The primary lesion was initially removed surgically, and the metastatic bone lesions and locally recurrent tumours were treated by a high-fractional dose and high-total-dose intensity-modulated radiotherapy (IMRT, 60 Gy at 2.5 Gy per fraction) without significant side effects. All the grossly relapsed tumors underwent complete remission (CR) within a short time after IMRT. To date, CR has been maintained for more than two years. This case study reports the successful treatment of radioresistant RCC using a new scheme that involves a fractionation regimen with a high precision radiotherapy.
For metastatic renal cell carcinomas (RCC), palliative radiotherapy is reported to be effective in providing the patient with symptom relief (1). However, RCC has historically been known to be resistant to conventional radiotherapy (2). RCC radioresistance is attributed to the slow regression of the tumor after radiotherapy (3). Therefore, radiosurgery has been performed to overcome the resistance of metastatic brain tumors to treatment (4). Radiosurgery is not easily applicable to extracranial tumors due to the limitation of immobilization and the potentially significant complications it may cause. Recently, intensity-modulated radiotherapy (IMRT) was developed and widely used in the clinic to deliver high doses to tumors while preserving the adjacent normal tissue. We report here that radioresponsiveness could be obtained in gross tumors originating from RCC, through IMRT by delivering a total dose of 60 Gy at 2.5 Gy fraction. This dosage was larger than that used in conventional fractionation (1.8-2.0 Gy). To our knowledge, this is the first report to show that renal fossa recurrence was salvaged successfully by IMRT.
A 56-yr-old female patient was referred with a mass in her kidney that was detected on ultrasonography during a regular health screen. Left RCC was detected by computed tomography (CT) (Fig. 1A). CT also revealed an osteolytic lesion in the left-lower ischial bone. In the whole-body bone scan, focal hot uptakes were detected in the left acetabulum and left anterior ilium as well as in the left ischium (Fig. 1B). A biopsy performed on the left ischial bone confirmed metastasis. The possibility that the hot uptake of the left acetabulum could be attributed to arthritic changes was high. However, it was difficult to rule out metastasis in the focal hot uptake of the left-anterior-ilium lesion. Radical nephrectomy was first performed on the left kidney (Fig. 1C). Histologically, the tumor showed conventional RCCa with Fuhrman nuclear grade 4 and focal sarcomatoid differentiation (pT1N0) (Fig. 1D, E). Seven lymph node samplings were negative.
After surgery, IMRT using simultaneously integrated boost (SIB-IMRT) was planned for the left pelvic bone (Fig. 2A). SIB-IMRT prescribes a different dose to a different target volume simultaneously. The basis of the treatment protocol was an irradiation in 24 fractions, with a total dose of 60 Gy (2.5 Gy per fraction) to the region of the macroscopic tumors, including the left ischium confirmed by biopsy and the possible metastatic lesion, a small, round osteoblastic lesion on the left anterior ilium. The protocol also involved a dose of 45 Gy (1.9 Gy per fraction) to the region of low-risk subclinical disease, including the rest of the left pelvic bone and excluding the high-dose region. A unique feature of the present SIB strategy is that irradiation of the microscopic tumors remains the same as in the conventional treatments, whereas the relapsed gross tumors are irradiated using a hypofractionation scheme. The IMRT plans were generated by the Corvus treatment planning system (NOMOS Corporation, Sewickley, PA, U.S.A.). The ischial bone gross tumor had almost disappeared by the follow-up study one month after IMRT (Fig. 3A).
Five months after IMRT to the left pelvic bone, new lesions that were suspected to be metastatic were detected in the right pelvis, and multiple conglomerated hypervascular solid masses had developed in the renal fossa. In addition, in the vicinity of the 9th-left-rib costochondral junction, an expansile mass was detected on the rib. As such, a second IMRT was performed on the newly detected metastasis and on the local recurrent lesions (Fig. 2B). Until two months after the second IMRT, systemic anti-cancer treatment had not been performed because of the patient's refusal. In the follow-up study that was conducted one month after radiation therapy alone, the rib expansile mass and the locally recurrent renal fossa mass underwent complete remission (CR) (Fig. 3). The patient tolerated both sessions of SIB-IMRT very well without any significant side effects or interruption.
After experiencing CR of all the grossly relapsed tumours, the patient accepted the systemic anti-cancer treatment. Hence, interferon-alpha systemic therapy (6,000,000 units, three times a week) was administered for four months. Subsequently, six months after the second IMRT, a small nodule, which had developed in the lower left lung approximately six months after surgery and thought to be merely an inflammatory nodule, showed a slight increase in size. Hence, under the suspicion of clinical metastasis (a histological test was performed, but failed to provide a definitive diagnosis of the condition), the patient is currently undergoing a clinical drug trial with Sunitinib (Sutent), given orally at a dose of 50 mg/day on a four-week-on/two-week-off schedule. Until now, the patient's locally and distantly relapsed lesions, which were treated with SIB-IMRT, have maintained their CR condition (Fig. 3) for more than two years.
Although the rib lesion in the vicinity of the left 9th costochondral junction might be salvaged even by 3D-conformal radiotherapy, IMRT could be essential to deliver high doses of radiation to the renal fossa close to the bowel without side effects. It could also be extremely useful for selectively delivering high dose radiation to the gross pelvic bone tumor and low dose radiation to the microscopic tumor in the remaining pelvis by the SIB technique. As a result, CRs were gained in all the gross RCC by high-precision IMRT alone, without any significant side effects.
More importantly, 60 Gy at 2.5 Gy per fractional treatment cleared all the gross lesions within 2-3 months, whereas using conventional fractionation (30-40 Gy) we generally experience minimal changes in the tumor size. We are planning to prove the efficacy of this prescription regimen in the prospective setting.
To our knowledge, this report is the first to show that in a renal fossa recurrence case, treatment with IMRT alone could lead to CR. Furthermore, the recurrent disease in the renal fossa has not been detected in the patient during the subsequent two years. After radical nephrectomy, renal fossa recurrence is very rare, but its prognosis is poor. Previous studies reporting local recurrence indicate many low-risk T1 cases. In all cases, the development of Fuhrman grades of tumors as well as various histological subtypes occurred. As such, the cause of renal fossa recurrence could not be determined (5-7). Instead, it could only be due to the biological heterogeneity of each tumor. Since it is very rare, it is usually described in case studies. Most reports outline the benefits of using an aggressive surgical approach for treatment (6, 8, 9). The optimal management has to be established on more experiences, considering side effects and treatment outcome of each modality including surgery, immunochemotherapy (ICT), and IMRT simultaneously (6, 8-10). The high-dose precision radiation therapy by IMRT may become the most important modality for the local control of RCC, overshadowing conventional radiation therapies.
To conclude, in metastatic and locally recurrent RCC, the IMRT technique, which prescribes a high-total-dose with a higher fractional dose than conventional treatment, gains CR in a short time, and maintains a sufficient disease-free state. IMRT also allows maximal preservation of the adjacent normal tissue. Thus, SIB-IMRT could be the most effective modality for the local control of RCC in patients that have locally and distantly relapsed gross tumors. In addition, SIB-IMRT allows RCC patients to preserve their quality of life with minimal side effects from the treatment.
Figures and Tables
Fig. 1
(A) Abdomino-pelvic CT revealing a heterogeneous mass lesion (approximately 7 cm in size) on the left kidney midpole extending to the renal pelvis. (B) Whole-body bone scan performed for staging workup, showing a focus of increased uptake in the left ischium, left anterior ilium, and left acetabulum. (C) Gross appearance: a circumscribed tumor in the midpole of the kidney shows a variegated cut surface composed of golden yellow and pinkish parenchyma and areas of necrosis. (D) This tumor is predominantly composed of conventional RCC (right side) and a small portion of sarcomatoid RCC (left side). The conventional RCC component has clear cells interspersed with abundant, thin-walled blood vessels, while the sarcomatoid component consists of interlacing bundles of spindle cells with pleomorphic nuclei. (E) Metastatic carcinoma on the ischial bone (H&E, ×200); most metastatic carcinoma are the sarcomatoid component of RCC.
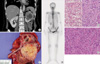
Fig. 2
Dose distribution for the SIB-IMRT plans. (A) The 1st IMRT SIB delivers 60 and 45.06 Gy in 24 fractions to the gross metastatic tumors (blue) and the rest of the left pelvic bone (violet). (B) The 2nd IMRT SIB also delivers 60 Gy in 24 fractions to the gross renal fossa recurrent tumor (blue) and the left rib mass in the vicinity of the left 9th costochondral junction (blue). The isodose lines of 60, 55, 50, 45, 30, and 20 Gy are shown.
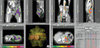
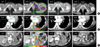
Fig. 3
Metastatic bony lesions to the left ischium (A), the left rib in the vicinity of the left 9th costochondral junction (B), and the renal fossa recurrent tumor (C). a-1, b-1, and c-1 reveal pre-treatment gross tumors. a-2, b-2, and c-2 show SIB-IMRT differential-dose distribution, which is highly conformed to the gross and microscopic tumors on each site. a-3, b-3, and c-3 are follow-up images showing complete disappearance 1-3 months after IMRT to each site. a-4 and b-4 show the re-ossification of the metastatic bones 20 months and 25 months after IMRT. c-4 also shows complete remission of renal fossa, which has been maintained 25 months after IMRT.
References
1. DiBiase SJ, Valicenti RK, Schultz D, Xie Y, Gomella LG, Corn BW. Palliative irradiation for focally symptomatic metastatic renal cell carcinoma: support for dose escalation based on a biological model. J Urol. 1997. 158:746–749.

2. Wronski M, Maor MH, Davis BJ, Sawaya R, Levin VA. External radiation of brain metastases from renal carcinoma: a retrospective study of 119 patients from the M. D. Anderson Cancer Center. Int J Radiat Oncol Biol Phys. 1997. 37:753–759.
3. Fertil B, Malaise EP. Intrinsic radiosensitivity of human cell lines is correlated with radioresponsiveness of human tumors: analysis of 101 published survival curves. Int J Radiat Oncol Biol Phys. 1985. 11:1699–1707.

4. Sheehan JP, Sun MH, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery in patients with renal cell carcinoma metastasis to the brain: long-term outcomes and prognostic factors influencing survival and local tumor control. J Neurosurg. 2003. 98:342–349.

5. Gogus C, Baltaci S, Beduk Y, Sahinli S, Kupeli S, Gogus O. Isolated local recurrence of renal cell carcinoma after radical nephrectomy: experience with 10 cases. Urology. 2003. 61:926–929.

6. Itano NB, Blute ML, Spotts B, Zincke H. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol. 2000. 164:322–325.

7. Schrodter S, Hakenberg OW, Manseck A, Leike S, Wirth MP. Outcome of surgical treatment of isolated local recurrence after radical nephrectomy for renal cell carcinoma. J Urol. 2002. 167:1630–1633.
8. Master VA, Gottschalk AR, Kane C, Carroll PR. Management of isolated renal fossa recurrence following radical nephrectomy. J Urol. 2005. 174:473–477.

9. Tanguay S, Pisters LL, Lawrence DD, Dinney CP. Therapy of locally recurrent renal cell carcinoma after nephrectomy. J Urol. 1996. 155:26–29.

10. Figlin RA, Abi-Aad AS, Belldegrun A, deKernion JB. The role of interferon and interleukin-2 in the immunotherapeutic approach to renal cell carcinoma. Semin Oncol. 1991. 18(5 Supple 7):102–107.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download