Abstract
The aims of this study were to determine the occurrence and variables associated with the initial intravenous immunoglobulin (IVIG) treatment failure in Kawasaki disease (KD) and to categorize differences in clinical characteristics between responders and nonresponders to initial IVIG treatment. Patients were classified into two groups. Group A included 33 patients who received a single dose of IVIG treatment and responded. Group B included 18 patients who received more than two doses of IVIG due to failure of the initial treatment. The mean duration of fever after initial treatment in group B was significantly longer than it was in group A. In group B, we found that higher bilirubin, aspartate aminotransferase (AST), polymorphonuclear cells (PMN) (%), and lower platelet values at baseline were independent predictors of persistent or recurrent fever in patients with KD. Coronary artery abnormalities were found in 8 patients (44.4%) in group B and in two patients (6.1%) in group A. We found that abnormal liver function tests and a lower platelet count at baseline were possible predictors of nonresponders to IVIG in patients with KD. There is a need for a prospective study focused on baseline hepatobiliary parameters.
Kawasaki disease (KD) is an acute inflammatory illness that affects young children and is characterized by persistent fever, various kinds of rashes, conjunctivitis, inflammation of mucous membranes, swollen erythematous hands and feet, and cervical lymphadenopathy (1, 2). The cause of KD is unknown; epidemiologic data suggest it may be an infectious agent, but intensive searches for such an agent have been unsuccessful. The use of intravenous immunoglobulin (IVIG) with aspirin is standard treatment and in most patients diminishes inflammation and vasculitis rapidly enough to prevent the development of coronary artery lesions (3). Coronary artery abnormalities such as aneurysms or ectasia develop in 3-5% of patients following treatment with IVIG and high-dose aspirin (4, 5). Without proper treatment, coronary artery aneurysms or ectasia develop in approximately 15% to 25% of affected children (6, 7). The administration of high-dose IVIG reduces both the duration of fever and the incidence of coronary artery aneurysms, but approximately 10-20% of patients have persistent or recurrent fever despite IVIG (8, 9). Some clinicians advocate re-treatment with IVIG or administration of pulsed steroids in children with persistent and recurrent fever or worsening echocardiography (8, 9). We retrospectively analyzed all children admitted with KD to determine the occurrence and variables associated with the initial IVIG treatment failure, and categorized differences in clinical characteristics between responders and nonresponders to initial IVIG treatment.
Patients who were admitted to the Department of Pediatrics, Kyung Hee University Hospital, Seoul, Korea between March 1995 and April 2004 and who satisfied the criteria for KD (10) were enrolled in this study. We included patients whose fever persisted more than 3 days who met other criteria, even though they did not meet all the clinical criteria initially. Our laboratory workup for patients with KD was a routine, retrospective laboratory analysis. All patients were initially treated with 2 g/kg IVIG during a 10-12 hr period, and aspirin (80-100 mg/kg/day in divided doses) was administered until the second week of treatment or until the fever subsided, after which it was reduced to 3-5 mg/kg/day.
Patients were classified into two groups. Group A included patients who received a single dose of IVIG treatment and responded (defined as defervescence by 48 hr after IVIG and no return of fever (>37.8℃) for at least 7 days after IVIG, with marked improvement or normalization of principal clinical findings) (8, 9). Group A included 33 patients who were admitted from January 2003 through April 2004. Group B included 18 patients who received more than two doses of IVIG due to failure of the initial treatment. This nonresponse was defined as the return of fever and one or more of the initial symptoms that led to the diagnosis of KD within 2 to 7 days of treatment with IVIG (8, 9). Eighteen patients who were admitted to our hospital between March 1995 and April 2004 were included in group B. We reviewed the clinical characteristics of KD patients in groups A and B, and we also analyzed laboratory parameters measured before and after the use of IVIG in both groups.
Two-dimensional echocardiography was performed at the time of diagnosis. These tests were repeated approximately at 1-2 weeks and at 7-10 weeks after diagnosis. Definitions of coronary dilatation and aneurysm were based on published criteria (11, 12): 1) a coronary artery luminal diameter of at least 3 mm in a child <5 yr old, or at least 4 mm in a child ≥5 yr; 2) an internal diameter of a segment at least 1.5 times as large as that of an adjacent segment; or 3) a clearly irregular lumen.
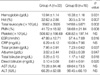
A total of 51 patients were enrolled in our study. Of these 51, 34 (66.7%) were boys. There were 33 patients in group A who received a single dose of IVIG, and 18 patients in group B who required more than two doses of IVIG. In group B, 4 patients did not initially respond and 14 others had return of fever and symptoms (2-7 days after initial treatment). Fifteen (83.3%) of the 18 patients in group B who required the second dose of IVIG responded well. The remaining 3 were treated with a third dose of IVIG and responded fully. Clinical characteristics of both groups are presented in Table 1. The mean duration of fever in group A was 10.36±8.24 hr after initial treatment, whereas it was 120.72±80.26 hr in group B. The mean duration of fever before initial treatment in group A was 150.91±79.41 hr, and in group B it was 123.58±55.92 hr. As we expected, the mean duration of fever after initial treatment in group B was significantly longer than it was in group A.
Laboratory results on admission for both groups are listed in Table 2. There were no differences between the groups in age, sex, hemoglobin, hematocrit, total leukocyte count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), total protein, albumin, alanine aminotransferase (ALT), or the Harada score before IVIG treatment. Although the total numbers of white blood cells (WBC) and hemoglobin values were not different between groups, group B had a higher percentage of polymorphonuclear cells (PMN) (80.83±10.24%, p=0.003), and a lower platelet count (326.67±100.68 × 103/µL, p=0.043) when compared with those (69.1±14.7%, 393.64±114.42 × 103/µL, respectively) of group A. Group B also had a higher serum total bilirubin level (2.75±2.13 mg/dL, p=0.000) when compared with that (1.26±1.24 mg/dL) of group A. Group B also had higher serum aspartate aminotransferase (AST) values (138.59±165.81 IU/L, p=0.027) when compared with that of group A (65.45±81.44 IU/L). γ-glutamyl transpeptidase (γ-GTP) and lactate dehydrogenase (LD) levels were excluded because the data in group B were insufficient. In our retrospective series of 18 patients in group B, we report that higher bilirubin, AST, PMN (%), and lower platelet values at baseline are independent predictors of persistent or recurrent fever in patients with KD. Laboratory results from both groups usually 48 hr after IVIG treatment are listed in Table 3. Contrary to the laboratory results before IVIG treatment, there were no differences between the groups in platelet count, AST, ALT, and CRP after the IVIG treatment. After IVIG treatment, group B had lower hemoglobin, higher total leukocyte counts, higher percentages of PMN, higher ESRs, lower total protein and albumin levels, and higher bilirubin levels. Somewhat contrary to previous studies, it seems that in both groups a higher percentage of PMN, lower platelet count, higher bilirubin level, and higher AST value were associated with the requirement for more than two doses of IVIG, before IVIG was administered. The high platelet count, a characteristic laboratory finding in KD, is not likely to be associated with additional IVIG treatment. Coronary artery abnormalities that developed during the acute phase and subacute phase were found in 8 patients (44.4%) in group B and in two patients (6.1%) in group A. In five of eight patients in group B, the abnormality developed in the acute phase, and in the other three it developed in the subacute phase. Six of eight patients who had coronary artery abnormalities improved in the convalescent phase. The remaining one patient did not receive a follow-up echocardiographic examination, and another patient still had a coronary aneurysm. There were two patients in group A who developed coronary artery aneurysms, and both returned to normal in the convalescent phase.
Since first reported in 1972, the number of cases of KD has increased continuously, including two epidemics, and thus KD is now recognized as an important and common childhood illness in our country (13). The average incidence of 86.4/100,000 children <5 yr old in South Korea is second only to that of 134.2 in Japan (14).
In spite of the effectiveness of IVIG treatment in the acute phase of KD (4, 5, 15), approximately 10-20% of patients experience a persistent or recurrent fever despite initial IVIG treatment (8, 9). Despite the use of standard IVIG regimens, fever persists for more than 3 days after treatment in 20-30% of children (5). These cases have been referred to as "nonresponders to IVIG", or "initial treatment failure", or "IVIG resistance", or "the re-treatment group" (4, 8, 15-20). A consensus definition is needed for future studies. The patients with persistent or recurrent fever could be at greatest risk of developing coronary artery aneurysms. It is certain that the febrile phase of the disease is significantly longer in children with coronary aneurysms (21, 22). If we could identify the factors related to patients with nonresponse to IVIG at admission, we could predict the need for subsequent re-treatment or the risk of subsequent development of aneurysm. We found that there were 18 patients who did not respond to initial doses of IVIG. The average proportion of nonresponders to IVIG in our study was 7.1% (2.2-11.5%) from 2001 to 2004. This is comparable to the previous findings of Sundel et al. (8) in 1993 (10%), Burns et al. (16) in 1998 (7.8%), Fukunishi et al. (17) in 2000 (15.9%), and Durongpisitkul et al. (18) in 2003 (11.6%).
A multicenter, retrospective study (16) concluded that persistent or recrudescent fever after the first course of IVIG was associated with an increased risk of treatment failure. The other definition of treatment failure, that of Wallace et al. (19), is the return of fever and one or more of the initial symptoms that led to the diagnosis of KD within 2 to 7 days of treatment with IVIG. They do not mention the development of coronary abnormalities. Coronary artery abnormalities that developed during the acute phase and subacute phase were found in eight patients (44.4%) among the nonresponders to IVIG (group B) and in two patients (6.1%) among the responders to IVIG (group A). These percentages are somewhat high compared with those in a previous paper (19) that reported that nearly 23% of patients with KD may require re-treatment and 8% may develop coronary aneurysm. However, Hashino et al. (15) in 2001 reported a high probability (48.6%) of developing coronary artery lesions in IVIG nonresponders, and Fukunishi et al. (17) in 2000 reported that the incidence of coronary artery aneurysm among nonresponders was 38.5%. There was no difference in age, sex, duration of symptoms prior to treatment, initial CRP, ESR, or the presence of an abnormal initial echocardiogram between initial responders and nonresponders (18, 19, 21). These findings are somewhat similar to our results in that there were no significant differences in age, sex, body-weight, and duration of fever before administration of IVIG between responders and nonresponders to IVIG. It is a natural result that the duration of fever after the initial IVIG administration was much longer in the nonresponding group than in the responding group. An interesting finding, which we did not analyze statistically, was the unusually high occurrence (94.4%) of cervical lymph node enlargement in the nonresponding group in spite of the fact that cervical lymphadenitis is the least common of the principal clinical features of KD.
We report that a higher bilirubin level, higher AST level, higher percentage of PMN, and a lower platelet count those that of the responding group at baseline were all independent predictors of persistent or recurrent fever in patients with KD. These patients could be given more IVIG. After IVIG treatment, group B had lower hemoglobin, higher total leukocyte counts, higher percentages of PMN, higher ESRs, lower total protein and albumin levels, and higher bilirubin levels. These significant differences in group B could be related to the suggestion of additional risk findings of the occurrence of coronary artery lesions. Many authors (4, 18, 23) have agreed that increased white blood cell and neutrophil counts and increased CRP concentration after IVIG treatment are risk factors for coronary artery lesions. However, we found no association of CRP concentration, which measured at admission and usually 48 hr after IVIG treatment, with failure of initial IVIG treatment. These results maybe associated with shorter febrile days before IVIG treatment compared with previous papers (17, 18, 29, 30) because of more or less low CRP levels. We found also no correlation between age, gender, and days since starting IVIG treatment, with failure of the initial IVIG treatment.
In the present study, a lower platelet count in the nonresponding group at baseline was an independent predictor of persistent or recurrent fever with possible coronary complications. Burns et al. (24) explained that the reason thrombocytopenia is associated with KD is that the increased platelet turnover occurs during the activation of coagulation in the early phase of the disease. Other parameters of anemia, left shift, and low platelet count secondary to increased platelet turnover presumably reflect increased release of inflammatory mediators and worsened vascular inflammation (25, 26). Fukunishi et al. (17) first reported that biliary tract-associated parameters such as total bilirubin and γ-GTP, which probably reflect inflammation of the bile duct, were not previously found to be associated with a high risk for development of coronary artery lesions. Also, the levels of AST/ALT were higher in nonresponders, but this was not clinically statistically significant. We had similar results in regard to baseline hepatobiliary parameters. Although we did not examine γ-GTP and lactate dehydrogenase levels, we found significantly elevated serum AST activity, and elevated direct and total bilirubin levels in the nonresponding group at the baseline examination. We found that serum ALT was elevated, but this difference was not statistically significant. Serum ALT activity was elevated in approximately one-third of KD patients, and 18% had levels exceeding 100 U/L. A case-comparison study (27) reported that elevated serum ALT activity was much more frequently observed at the time of initial evaluation and diagnosis of KD.
A nationwide epidemiologic study in Japan (28) demonstrated a relationship between the level of serum transaminase and the development of cardiac disorders. The elevation of the ALT in KD patients is associated with the development of cardiac disorders. The proportion of those without cardiac disorders with an ALT of 50 IU/I or more was 36%. An ALT of 50 IU/I or more was noted in 49% of the patients with transient cardiac conditions and 52% of those with persistent cardiac disorders. It was proved that the elevation of the serum ALT at the initial examination was related to the complications of both transient and persistent cardiac disorders.
In conclusion, we found that a higher bilirubin level, higher AST level, higher percentage of PMN, and lower platelet count than those of the responder group at baseline were independent predictors of persistent or recurrent fever in patients with KD. The patients who did not respond to initial IVIG treatment had a higher probability (44.4%) of developing coronary artery abnormalities. The optimal re-treatment dose of IVIG and treatment guidelines should be determined for this small group of nonresponders to IVIG. There is a need for a prospective study focused on baseline hepatobiliary parameters.
Figures and Tables
References
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967. 16:178–222.
2. Kawasaki T, Kosaki F, Okawa S, Shigematsu I, Yanagawa H. A new infantile acute febrile mucocutaneous lymph node syndrome (MLNS) prevailing in Japan. Pediatrics. 1974. 54:271–276.
3. Durongpisitkul K, Gururaj VJ, Park JM, Martin CF. The prevention of coronary artery aneurysm in Kawasaki disease: a meta-analysis of the efficacy of aspirin and immunoglobulin treatment. Pediatrics. 1995. 96:1057–1061.
4. Newburger JW, Takahashi M, Burns JC, Beiser AS, Chung KJ, Duffy CE, Glode MP, Mason WH, Reddy V, Sanders SP, Shulman ST, Wiggins JW, Hicks RV, Fulton DR, Lewis AB, Leung DY, Colton T, Rosen FS, Melish ME. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986. 315:341–347.

5. Newburger JW, Takahashi M, Beiser AS, Burns JC, Bastian J, Chung KJ, Colan SD, Duffy CE, Fulton DR, Glode MP, Mason WH, Meissner HC, Rowley AH, Shulman ST, Reddy V, Sundel RP, Wiggins JW, Colton T, Melish ME, Rosen FS. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N Engl J Med. 1991. 324:1633–1639.

6. Kato H, Ichinose E, Yoshioka F, Takechi T, Matsunaga S, Suzuki K, Rikitake N. Fate of coronary aneurysms in Kawasaki disease: serial coronary angiography and long-term follow-up study. Am J Cardiol. 1982. 49:1758–1766.

7. Suzuki A, Kamiya T, Kuwahara N, Ono Y, Kohata T, Kimura K, Takamiya M. Coronary arterial lesions of Kawasaki disease: cardiac catheterization findings of 1100 cases. Pediatr Cardiol. 1986. 7:3–9.

8. Sundel RP, Burns JC, Baker A, Beiser AS, Newburger JW. Gamma globulin re-treatment in Kawasaki disease. J Pediatr. 1993. 123:657–659.

9. Wright DA, Newburger JW, Baker A, Sundel RP. Treatment of immune globulin-resistant Kawasaki disease with pulse doses of corticosteroids. J Pediatr. 1996. 128:146–149.
10. Morens DM, O'Brien RJ. Kawasaki disease in the United States. J Infect Dis. 1978. 137:91–93.
11. Arjunan K, Daniels SR, Meyer RA, Schwartz DC, Barron H, Kaplan S. Coronary artery caliber in normal children and patients with Kawasaki disease but without aneurysms: an echocardiographic and angiographic study. J Am Coll Cardiol. 1986. 8:1119–1124.

12. Takahashi M, Mason W, Lewis A. Regression of coronary artery aneurysms in patients with Kawasaki syndrome. Circulation. 1987. 75:387–394.
13. Park YW, Han JW, Park IS, Kim CH, Yun YS, Cha SH, Ma JS, Lee SB, Kim CH, Lee HJ, Tockgo YC. Epidemiologic picture of Kawasaki diseases in Korea, 2000-2002. Pediatr Int. 2005. 47:382–387.
14. Newburger JW, Taubert KA, Shulman ST, Rowley AH, Gewitz MH, Takahashi M, McCrindle BW. Summary and Abstracts of the Seventh International Kawasaki Disease Symposium: December 4-7, 2001, Hakone, Japan. Pediatr Res. 2003. 53:153–157.

15. Hashino K, Ishii M, Iemura M, Akagi T, Kato H. Re-treatment for immune globulin-resistant Kawasaki disease: a comparative study of additional immune globulin and steroid pulse therapy. Pediatr Int. 2001. 43:211–217.

16. Burns JC, Capparelli EV, Brown JA, Newburger JW, Glode MP. Intravenous gamma-globulin treatment and retreatment in Kawasaki disease. Pediatr Infect Dis J. 1998. 17:1144–1148.

17. Fukunishi M, Kikkawa M, Hamana K, Onodera T, Matsuzaki K, Matsumoto Y, Hara J. Prediction of non-responsiveness to intravenous high-dose γ-globulin therapy in patients with Kawasaki disease at onset. J Pediatr. 2000. 137:172–176.

18. Durongpisitkul K, Soongswang J, Laohaprasitiporn D, Nana A, Prachuabmoh C, Kangkagate C. Immunoglobulin failure and retreatment in Kawasaki disease. Pediatr Cardiol. 2003. 24:145–148.

19. Wallace CA, French JW, Kahn SJ, Sherry DD. Initial intravenous gammaglobulin treatment failure in Kawasaki disease. Pediatrics. 2000. 105:E78.

20. Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, Shulman ST, Bolger AF, Ferrieri P, Baltimore RS, Wilson WR, Baddour LM, Levison ME, Pallasch TJ, Falace DA, Taubert KA. Diagnosis, treatment, and long-term management of Kawasaki disease. A statement for Health Professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004. 110:2747–2771.

21. Koren G, Lavi S, Rose V, Rowe R. Kawasaki disease: review of risk factors for coronary aneurysms. J Pediatr. 1986. 108:388–392.

22. Daniels SR, Specker B, Capannari TE, Schwartz DC, Burke MJ, Kaplan S. Correlates of coronary artery aneurysm formation in patients with Kawasaki disease. Am J Dis Child. 1987. 141:205–207.

23. Mori M, Imagawa T, Yasui K, Kanaya A, Yokota S. Predictors of coronary artery lesions after intravenous γ-globulin treatment in Kawasaki disease. J Pediatr. 2000. 137:177–180.

24. Burns JC, Glode MP, Clarke SH, Wiggins J Jr, Hathaway WE. Coagulopathy and platelet activation in Kawasaki syndrome: Identification of patients at high risk for development of coronary artery aneurysms. J Pediatr. 1984. 105:206–211.

25. Lang BA, Silverman ED, Laxer RM, Rose V, Nelson DI, Rubin LA. Serum-soluble interleukin-2 receptor levels in Kawasaki disease. J Pediatr. 1990. 116:592–596.

26. Lin CY, Lin CC, Hwang B, Chiang BN. The changes of interleukin-2, tumor necrotic factor and gamma-interferon production among patients with Kawasaki disease. Eur J Pediatr. 1991. 150:179–182.
27. Burns JC, Mason WH, Glode MP, Shulman ST, Melish ME, Meissner C, Bastian J, Beiser AS, Meyerson HM, Newburger JW. Clinical and epidemiologic characteristics of patients referred for evaluation of possible Kawasaki disease. J Pediatr. 1991. 118:680–686.

28. Uehara R, Yashiro M, Hayasaka S, Oki I, Nakamura Y, Muta H, Ishii M, Matsuishi T, Sonobe T, Yanagawa H. Serum alanine aminotransferase concentrations in patients with Kawasaki disease. Pediatr Infect Dis J. 2003. 22:839–842.

29. Lee GB, Lee JW, Lee KY. Prediction of intravenous immunoglobulin non-responders in patients with Kawasaki disease. Korean J Pediatr. 2004. 47:90–94.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download