Abstract
The aim of this study was to investigate the possible adverse effects of Asian dust events on respiratory health in asthmatic children. Fifty-two children with mild asthma were studied for eight consecutive weeks in the spring of 2004 (March 8 to May 2). During the study period, five Asian dust days were identified; we included a lag period of two days following each of the events. Subjects recorded their respiratory symptom diaries and peak expiratory flow (PEF) twice daily during the study period; and they underwent methacholine bronchial challenge tests. The subjects reported a significantly higher frequency of respiratory symptoms during the Asian dust days than during the control days. They showed significantly more reduced morning and evening PEF values, and more increased PEF variability (10.1%±3.5% vs. 5.5%±2.2%) during the Asian dust days than during the control days. Methacholine PC20 was not significantly different between before and after the study period (geometric mean: 2.82 mg/mL vs. 3.16 mg/mL). These results suggest that the short-term Asian dust events might be associated with increased acute respiratory symptoms and changes in PEF outcomes. However, there might be little long-term influence on airway hyperresponsiveness in children with mild asthma.
Wind-blown dust originating from the arid deserts of Mongolia and China, so called Asian dust, is a well-known springtime meteorological phenomenon in Korea (1). Public concerns about the possible adverse effects of this dust have increased because the occurrence of these dust events has become more frequent and more erratic during the last decade (2).
Asian dust events are a form of air pollution episode. Exposure to ambient air pollutants has been associated with the impairment of respiratory health (3). Results of epidemiologic studies have shown that asthmatic subjects are more susceptible to air pollutants than are other groups (4, 5). A recent study has reported altered respiratory symptoms and peak expiratory flow (PEF) outcomes during the Asian dust events in adult asthmatic patients (6). However, little is known about their effects on asthmatic children.
The severity of asthma has been assessed based on symptom diary recordings and measurements of pulmonary function. Home PEF monitoring has been widely used for the monitoring of asthma in both epidemiological studies and clinical trials (7).
The aims of this study were to investigate the possible effects of Asian dust events on respiratory symptoms and pulmonary function by measuring PEF and to estimate the changes of airway hyperresponsiveness (AHR) after exposure to Asian dust events in asthmatic children.
A group of 52 children with mild asthma were recruited from the allergy clinic at Seoul National University Children's Hospital. All subjects had a history of wheezing and dyspnea, and a methacholine PC20 (provocative concentration causing a 20% fall in forced expiratory volume in one second [FEV1]) of less than 16 mg/mL. The clinical severity of asthma was assessed according to the National Asthma Education and Prevention Program (8). The subjects whose clinical severity could not be accurately assessed were excluded. All patients lived in the Seoul Metropolitan area during the study period. Atopy was defined as the presence of at least one positive skin prick test result to a panel of 13 common aeroallergens, in the presence of positive and negative controls. The allergens tested were: 1) house dust mites (Dermatophagoides pteronyssinus and Dermatophagoides farinae), 2) animal danders (cat epithelium and dog epithelium), 3) pollens (mugwort, ryegrass, ragweed, hazel, alder, and oak), 4) molds (Aspergillus fumigatus and Alternaria alternata), and 5) cockroach (Blatella germanica). Parents provided written informed consent for their children to participate in the study.
At the beginning of the study, parents and children were instructed to complete diaries twice daily with regard to respiratory symptoms. The current day symptom scores reflected respiratory symptoms experienced that day (presence or absence) and the nighttime records associated with the preceding night's symptoms (filled out each morning). The number of as-needed short-acting bronchodilator use was also noted.
Participants were asked to record PEF each morning and each evening using a Mini-Wright adult type peak-flow meter (Clement Clarke Ltd., London, U.K.). After achieving documented reproducibility within 20 L/min, PEF recordings were taken twice daily for eight consecutive weeks (between March 8 and May 2). Morning values were obtained at 8:00 a.m. and evening values at 8:00 p.m. On each occasion, the best of three attempts was recorded. Subjects inhaled two doses of 200 µg salbutamol sulfate (Ventolin, Glaxo, U.K.) immediately afterwards, and all these measurements were repeated 15 min later. During the study, asthmatic patients were asked to refrain from taking any medication other than salbutamol sulfate, 200-400 µg, which was allowed as a rescue medication, but preferably not within 4 hr before the PEF measurements. At the time of the study, all patients were free of acute respiratory tract infections or asthma exacerbations for 4 weeks. All patients were asked to discontinue oral theophylline for 48 hr, long-acting β2-agonists for 48 hr, and inhaled corticosteroids for 7 days before the start of the study. Diary cards with values missing for more than 10% of the eight weeks were excluded from the final analysis.
Methacholine bronchial challenges were carried out using a modification of the method described by Chai et al. (9). Concentrations (0.075, 0.15, 0.3, 0.625, 1.25, 2.5, 5, 10, and 25 mg/mL) of methacholine (Sigma-Aldrich, St Louis, MO, U.S.A.) were prepared by diluting with buffered saline (pH 7.4). A Rosenthal-French dosimeter (Laboratory for Applied Immunology, Baltimore, MD, U.S.A.) triggered by a solenoid valve set to remain open for 0.6 sec was used to deliver aerosol generated from a DeVilbiss 646 nebulizer with pressurized air at 20 psi. Each subject inhaled five inspiratory capacity breaths of buffered saline and increasing concentrations of methacholine until the FEV1 fell by >20% of the post-saline value. FEV1 was measured 1.5 min after each concentration was inhaled. The methacholine PC20 was obtained from the log concentration-percent fall in FEV1 curve by linear interpolation of the last two points. These tests were repeated within 1 month after the study period.
Daily information on Asian dust events during the study period was provided by the Korea Meteorological Administration from a station located in central Seoul. During the study period, five Asian dust days (March 10-11, March 30-31, and April 23) were identified in the Seoul Metropolitan area.
There were five Asian dust days during the length of the study, and the effects of dust events, if any, might have been delayed or manifested over a period of several days. To investigate potential effects between exposure and outcomes (time lapse), we examined a lag period of two days following each of the events (10, 11). The control days included all the days during the study period except the Asian dust days and the lag periods. Average levels of respiratory symptoms and pulmonary function parameters on the Asian dust days and during the lag period were compared with those of the control days. Daily symptom prevalence was calculated as the fraction (%) of children reporting symptoms or medication use, using data only from children with complete dairy information for each separate day. The proportion of decrements larger than 10% of morning PEF was calculated as the number of children experiencing a decrement divided by the total number of children reporting valid PEF measurements on each day of the study. The percentage of evening decrements was calculated in the same way. PEF diurnal variability, which was equal to the difference between morning and afternoon PEF values divided by the mean value, was calculated. Statistical comparisons of average daily reported respiratory symptoms and the PEF indices between each period were made with the Kruskall-Wallis non-parametric test. Correlation between daily average PM10 concentration and PEF variability was examined using Pearson correlation tests. Methacholine PC20 values obtained before and after the study period were log-transformed for statistical analysis, and the difference between the two was calculated by the paired t-test. All data analyses were performed using SPSS version 12.0 (SPSS Inc, Chicago, IL, U.S.A.). In each case, statistical significance was accepted when the two-sided p value was <0.05.
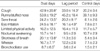
Of the 57 patients who were initially enrolled in this study, five were excluded because of unacceptable PEF records or having symptoms suggestive of respiratory infections during the study period. The characteristics of the 52 study subjects are shown in Table 1. The mean age of 32 boys and 20 girls was 10.5±3.2 yr. Atopy was identified in 45 (86.5%) subjects.
The daily prevalence of acute respiratory symptoms and signs was significantly higher during the Asian dust days than during the control days (p<0.05). More as-needed bronchodilator use was noted during the Asian dust days than during the lag period (p=0.016). Regarding the lag period, the daily prevalence of cough, sore throat, or eye irritation was significantly higher during the lag period than during the control days (p<0.05) (Table 2).
Table 3 summarizes the PEF parameters during each period. The mean morning PEF value (87.9±5.2%) and evening PEF value (90.4±5.4%) for % best during the Asian dust days were significantly (p<0.05) lower than those during the control days (morning, 94.2±3.2%; evening, 92.8±4.3%). The mean proportion of decrements larger than 10% of morning PEF was higher during the Asian dust days (57.3±16.0%) than during the control days (21.4±5.5%, p=0.015). The evening data showed similar results (40.7±18.3% vs. 21.0±4.3%, p=0.023). PEF diurnal variability during the Asian dust days was significantly higher than during the control days (10.1±3.5% vs. 5.5±2.2%, p=0.017), and it was augmented when post-bronchodilator values were included (15.8±6.8% vs. 10.9±7.2%, p=0.011). All PEF indices except the proportion of decrements of larger than 10% of evening PEF during the Asian dust days were significantly different from those during the lag period. There was a significant association between the degree of average PM10 concentration and PEF diurnal variability during the study period (r=0.778, p=0.007) (Fig. 1).
Among the 52 subjects with a measurable PC20 both before and after the study period, five subjects exhibited an increase in PC20 and five subjects showed a decrease in PC20 by a twofold magnitude (Fig. 2). The geometric mean of methacholine PC20 after the study period (3.16 mg/mL) was not significantly different from that before the study period (2.82 mg/mL, p=0.290).
This study demonstrated that children with mild asthma reported more respiratory symptoms, as needed bronchodilator use, and changes in PEF outcomes during the Asian dust days than during the control days. Our findings are consistent with previous data that have revealed associations between respiratory outcomes and Asian dust (6, 12, 13). Although several measures of respiratory symptoms were significantly increased up to the lag period, these findings appeared to be mostly transient.
The changes in airway caliber can be reliably monitored by home recordings of PEF over a period of time (7). We found decrements of both morning and evening PEF values and increases in the diurnal variability during the Asian dust days. The reduction of evening PEF might have resulted from the preceding daytime Asian dust event. However, morning decrements in PEF on the first Asian dust day may appear to be an unusual finding, because changes in morning values are not a result from the current day of Asian dust exposure. This might be explained as a possible effect of the presence of the Asian dust before notification from the Meteorological Administration was publicly made. The reduction in PEF values could partly explain the increase in diurnal variability during the Asian dust days, assuming parallel changes in morning and evening values (14).
We have shown that the effects of the Asian dust lasted at least two days after the exposure occurred. Several measures of respiratory symptoms and PEF parameters during the lag period were significantly different from those during the control days. The findings in this study are consistent with other epidemiologic studies that have suggested an association with air pollution and delayed manifestation of respiratory effects (10, 11), indicating that pollutant-induced inflammation may play a role in these relations (10).
An estimation of changes in bronchial reactivity in our study subjects was made before and after the study period. The initial values of methacholine PC20 were not significantly different from those after the eight-week study period. For patients with asthma, a change of at least one doubling concentration in bronchial sensitivity to a bronchoconstrictor stimulus is considered to be clinically significant in the evaluation of disease progression (15). For the patients in this study, most were unchanged on the basis of this criterion, and when changes occurred, their frequency was evenly distributed in either direction. The overall unchanged AHR, before and after the study period, indicates that the Asian dust events may have little or no long-term effects on airway reactivity in children with mild asthma. However, these findings must be interpreted with caution because there was a lower concentration of the Asian dust during the study period than that observed in preceding years. Also, these observations could partly be explained by patients selection. In this study, subjects with mild asthma were chosen in order to avoid severe exacerbation during the study period. The difference between before and after study period might have been greater if the measurements had been done including subjects with moderate to severe asthma.
Although exposure to an Asian dust event was associated with an altered respiratory outcome (6), the exact mechanism by which the dust causes adverse respiratory health effects is unclear. One possible explanation is an increase in the concentration of fine or ultrafine particles during the Asian dust events (16). Fine particles are of the greatest health concern because they can be inhaled deeply into the lungs (16). A further possibility is that the aerosol properties during Asian dust events were different from those of the general atmospheric conditions. It has been shown that chemical components in the atmosphere during Asian dust event included NO2, SO2, and O3, (17) and that they may produce adverse effects on the pulmonary function and respiratory symptoms both in asthmatics and normal subjects (11, 18, 19).
Several limitations should be considered when interpreting our results. We observed consistent effects of the Asian dust on respiratory health in this panel of mainly asthmatic children. As pollutant exposure was common to all members of the cohort, a traditional "control" group was not needed; each subject acted as his or her own control, and only covariates that varied across time within an individual needed to be considered for analysis (20). The Asian dust might not be adequately characterized by fixed-site ambient air concentrations, since children spend a varied portion of their time outdoors (21). Misclassification of the records of respiratory symptoms and PEF measurements in children by their parents is also possible. This may be overestimated because parents of asthmatic children are very aware of their children's respiratory health. Therefore, knowledge of Asian dust events by parents might affect symptom records or PEF measurements (22). Given that respiratory infections are related to asthma, they might have confounded the observed associations. However, we took respiratory infections into account for analysis, and this inclusion did not substantially alter the association between the Asian dust and respiratory outcomes. It is also possible that the sensitization to pollen may produce respiratory symptoms and change PEF indices. However, this is thought to be unlikely because this effect might exist not only in the control days but also in the Asian dust days.
In conclusion, our mild asthmatic children showed increased upper and lower respiratory symptoms, as needed bronchodilator use, and changes in PEF outcomes during the Asian dust days, which might also have delayed effects. Although several asthmatic children had considerable individual changes in AHR, as a group they remained similar over time. These findings indicate that Asian dust events increase the risk of acute respiratory symptoms and pulmonary function deterioration but do not appear to have long-term influence on AHR in children with mild asthma. Because we did not attempt to estimate these effects in subjects with moderate to severe asthma, the actual relations between the Asian dusts and respiratory outcomes may be greater than those observed in our study.
Figures and Tables
 | Fig. 1Daily average of PM10 concentration (-•-, µg/m3) and peak expiratory flow (PEF) variability (▨) in Seoul, Korea in the spring of 2004. |
 | Fig. 2Methacholine PC20 values of the asthmatic children before and after the study period. Horizontal bars represent the geometric mean and its range of 1SD. |
References
1. Kwon HJ, Cho SH, Chun Y, Lagarde F, Pershagen G. Effects of the Asian dust events on daily mortality in Seoul, Korea. Environ Res. 2002. 90:1–5.

2. Hwang SS, Cho SH, Kwon HJ. Effects of the severe Asian dust events on daily mortality during the spring of 2002, in Seoul, Korea. J Prev Med Pub Health. 2005. 38:197–202.
3. Schwartz J, Dockery DW, Neas LM, Wypij D, Ware JH, Spengler JD, Koutrakis P, Speizer FE, Ferris BG Jr. Acute effects of summer air pollution on respiratory symptom reporting in children. Am J Respir Crit Care Med. 1994. 150:1234–1242.

4. Vedal S, Petkau J, White R, Blair J. Acute effects of ambient inhalable particles in asthmatic and nonasthmatic children. Am J Respir Crit Care Med. 1998. 157:1034–1043.

5. Preutthipan A, Udomsubpayakul U, Chaisupamongkollarp T, Pentamwa P. Effect of PM10 pollution in Bangkok on children with and without asthma. Pediatr Pulmonol. 2004. 37:187–192.
6. Park JW, Lim YH, Kyung SY, An CH, Lee SP, Jeong SH, Ju YS. Effects of ambient particulate matter on peak expiratory flow rates and respiratory symptoms of asthmatics during Asian dust periods in Korea. Respirology. 2005. 10:470–476.

7. Reddel HK, Salome CM, Peat JK, Woolcock AJ. Which index of peak expiratory flow is most useful in the management of stable asthma? Am J Respir Crit Care Med. 1995. 151:1320–1325.

8. National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma expert panel. Report 2. 1997. Bethesda, Md: US Dept of Health and Human Services;NIH publication 97-4051.
9. Chai H, Farr RS, Froehlich LA, Mathison DA, McLean JA, Rosenthal RR, Sheffer AL, Spector SL, Townley RG. Standardization of bronchial inhalation challenge procedures. J Allergy Clin Immunol. 1975. 56:323–327.

10. Romieu I, Meneses F, Ruiz S, Huerta J, Sienra JJ, White M, Etzel R, Hernandez M. Effects of intermittent ozone exposure on peak expiratory flow and respiratory symptoms among asthmatic children in Mexico City. Arch Environ Health. 1997. 52:368–376.

11. Ostro B, Lipsett M, Mann J, Braxton-Owens H, White M. Air pollution and exacerbation of asthma in African-American children in Los Angeles. Epidemiology. 2001. 12:200–208.
12. Park JW, Lim YH, Kyung SY, An CH, Lee SP, Jeong SH, Ju YS. Effects of ambient particulate matter (PM10) on peak expiratory flow and respiratory symptoms in subjects with bronchial asthma during Yellow Sand period. Tubercul Respir Dis. 2003. 55:570–578.
13. Min PK, Kim CW, Yun YJ, Chang JH, Chu JK, Lee KE, Han JY, Park JW, Hong CS. Effect of Yellow Sand on respiratory symptoms and diurnal variation of peak expiratory flow in patients with bronchial asthma. J Asthma Allergy Clin Immunol. 2001. 21:1179–1186.
14. Hakala K, Stenius-Aarniala B, Sovijarvi A. Effects of weight loss on peak flow variability, airways obstruction, and lung volumes in obese patients with asthma. Chest. 2000. 118:1315–1321.

16. Lei YC, Chan CC, Wang PY, Lee CT, Cheng TJ. Effects of Asian dust event particles on inflammation markers in peripheral blood and bronchoalveolar lavage in pulmonary hypertensive rats. Environ Res. 2004. 95:71–76.

17. Choi JC, Lee MH, Chun YS, Kim J, Oh S. Chemical composition and source signature of spring aerosol in Seoul Korea. J Geophys Res. 2001. 106:18067–18074.

18. Blomberg A, Krishna MT, Bocchino V, Biscione GL, Shute JK, Kelly FJ, Frew AJ, Holgate ST, Sandstrom T. The inflammatory effects of 2 ppm NO2 on the airways of healthy subjects. Am J Respir Crit Care Med. 1997. 156:418–424.
19. Burnett RT, Smith-Doiron M, Stieb D, Raizenne ME, Brook JR, Dales RE, Leech JA, Cakmak S, Krewski D. Association between ozone and hospitalization for acute respiratory diseases in children less than 2 years of age. Am J Epidemiol. 2001. 153:444–452.

20. Ward DJ, Ayres JG. Particulate air pollution and panel studies in children: a systemic review. Occup Environ med. 2004. 61:e13.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download