Abstract
Despite the wide acceptance of endovascular aneurysmal repair in patients with abdominal aortic aneurysm (EVAR), stringent morphologic criteria recommended by manufacturers may preclude this treatment in patients with AAA. The purpose of this study was to investigate how many patients are feasible by Zenith and Excluder stent graft system, which are available in Korea. Eighty-two AAA patients (71 men, mean age 70 yr) who had been treated surgically or medically from January 2005 to December 2006 were included. Criteria for morphologic suitability (MS) were examined to focus on characteristics of aneurysm; proximal and distal landing zone; angulation and involvement of both iliac artery aneurysms. Twenty-eight patients (34.1%) were feasible in Zenith stent graft and 31 patients (37.8%) were feasible in Excluder. The patients who were excluded EVAR had an average of 1.61 exclusion criteria. The main reasons for exclusion were an unfavorable proximal neck (n=34, 41.5%) and problem of distal landing zone (n=25, 30.5%). There was no statistical significance among gender, age or aneurysm size in terms of MS. Only 32 patients (39%) who had AAA were estimated to be suitable for two currently approved grafts by strict criteria. However, even unfavorable AAA patients who have severe co-mobidities will be included in EVAR in the near future. Therefore, more efforts including fine skill and anatomical understanding will be needed to meet these challenging cases.
Since endovascular aneurysm repair (EVAR) was introduced by Parodi et al. in 1991 (1), EVAR is widely accepted as the alternative treatment to open repair for abdominal aortic aneurysm (AAA) (2-9) and is available in Korea (10). Although long-term results are not available, several reports have demonstrated the advantages of EVAR including a lower 30-day mortality, reduced operation time, shorter hospital stay, and quicker return to normal activity compared to open repair (4, 6-8). However, 14-66% of AAA patients are eligible for EVAR according to several reports from western countries in terms of morphologic suitability (MS) (11-13). To our knowledge, there has been no study regarding MS in patients with AAA in Korea. This study may be important because improper application against anatomical factors will be related with higher rates of complications and clinical failure, such as endoleak, mesenteric ischemia, or rupture (2, 14, 15). The aim of this study was to examine MS of EVAR with currently available grafts and to obtain basic information to improve the outcome by better patient selection.
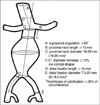
We reviewed the computed tomographic angiography (CTA) of 82 patients that had been diagnosed as AAA and were managed medically (7 patients) and surgically (open repair in 66, EVAR in 9) from January 2005 to December 2006 in Seoul National University Hospital and Seoul National University Bundang Hospital. Inclusion criteria of Zenith (Cook Company, Bloomington, IL, U.S.A.) and Excluder (WL Gore, Flagstaff, AZ, U.S.A.) that is available in Korea were adopted to analyze suitability (Fig. 1). Referring to the manufacturer's recommendations, a single observer reviewed the CTA images of each patient and calculated the following numeric data to be required for estimating MS with electronic caliper in PACS (M-View™, Marotech, Seoul, Korea); proximal neck length; proximal neck diameter; maximal diameter of aneurysm; diameter of common iliac and external iliac artery (EIA); diameter of common femoral artery; distal fixation length. The shortest diameter was measured from outer wall to outer wall in Zenith and from inner wall to inner wall in Excluder. Angle was measured as the most severe angle between the longitudinal axis of aneurysm body and proximal aortic neck in 3D reconstruction. Proximal neck length was calculated from the lowest margin of caudal renal artery to the onset of aneurysm considering the slice thickness. Briefly, aortic neck volumetry was performed by manually segregating each single consecutive axial CTA slice between start and stop slices and calculated. Distal fixation length was considered as the length of fixation site deliberating sufficient sealing and the origin of internal iliac artery (IIA). For example, if aneurysm was confined to only the proximal common iliac artery (CIA), EVAR would be eligible in the routine manner. However, if the aneurysm involvement extended to the origin of IIA, endograft limb should be deployed in EIA, which occludes the IIA. The morphologic suitability and the reason for exclusion were examined. The differences between suitable and unsuitable population was analyzed with Students t-test and χ2-test, and Pearson's correlation.
Among 82 patients with infrarenal AAA, there were 71 men (86.6%) and 11 women with mean age of 70 yr old (range, 50-87). The characteristics of AAA were summarized in Table 1. Maximum diameter of AAA was 60 ± 13 mm. Combined CIA aneurysm (diameter ≥ 18 mm) was found in 49 patients (59.8%) at left side, 52 patients (63.4%) at right side, and 45 patients (54.9%) at both sides. One patient (1.2%) was found to have both CIA aneurysm extending to both EIA and both IIA (Fig. 2A). Solitary EIA aneurysm was not found. With respect to IIA aneurysm, 5 patients (6.1%) had left IIA aneurysm, 4 patients (4.9%) right and 8 patients (9.8%) both. Diameter of left and right distal EIA was 11.49 ± 1.74 mm and 11.69 ± 1.55 mm. According to EUROSTAR classification for AAA (16, there were type A in 14 (17.1%), type B in 13 (15.9%), type C in 13 (15.9%), type D in 20 (24.4%), and type E in 22 patients (26.8%).
When we applied the manufacturer's criteria, EVAR was not able to perform in 48 patients (58.5%) in Zenith and 46 patients (56.1%) in Excluder. The main reason of exclusion was proximal neck problem (Zenith; 37.8%, Excluder; 41.5%). If both CIA aneurysms were treated with exclusion of IIA, distal fixation problem occurred in 9 patients with Zenith and in 11 patients with Excluder. However, as exclusion of both IIA would induce pelvic ischemia, we considered that exclusion criteria should be added with both CIA aneurysms, therefore the distal fixation problem was increased from 9 patients to 25 patients in both groups (Table 2). In summary, when we simply, followed the recommendations 41.5% of patients would be suitable in Zenith and 43.9% in Excluder, so 46.3% of overall MS would be estimated. However, if we adopt both CIA aneurysm requiring bilateral IIA embolization as another exclusion criterion, only 39% of patients should be considered suitable (data not shown). The patients who were excluded for EVAR had an average of 1.61 exclusion criteria (1-5).
Statistically significant differences between suitable and unsuitable population were shown in the suprarenal angle (the angle between suprarenal aorta and infrarenal aortic neck), infrarenal angle (the angle between infrarenal aortic neck and the aneurysm axis) and both CIA dilation in Zenith (p=0.021, 0.048, 0.001, 0.004). So was the infrarenal angle and Lt. CIA dilation in Excluder (p=0.049, 0.012). In overall MS, the infrarenal angle and both CIA dilation was estimated as having a statically significance (p=0.040, 0.005, 0.030). However, aneurysm neck length itself does not provide a statistical significance (p=0.187 in Zenith, p=0.404 in Excluder). Sex, age (>70 yr), maximum diameter of aneurysm (>60 mm) did not show a statistical significance about overall morphologic suitability by χ2-test (p=0.325, OR, 2.077; p=0.494, OR, 1.500; p=0.072, OR, 0.419) (p<0.05).
When we correlated each anatomical factor to disclose feature of AAA, there was some statistically significance by Pearson's correlation. With respect to characteristics of aneurysm, we interpreted the correlation as follows:
1) The older the patients, the more severe the tortuosity of aneurysm (p=0.043).
2) The more severe the suprarenal angle, the more severe the infrarenal angle (p=0.0001).
3) The more increase of the maximum diameter of AAA, the more increase of neck tortuosity and the higher occurrence of CIA aneurysm (p=0.017).
Since EVAR was first introduced by Parodi et al. in 1991 (1), there are some controversies about the therapeutic role of EVAR due to the absence of long-term outcome data (4-6). However, clinical trials of EVAR are increasing worldwide now (2-9). According to the national survey from the Korean society for vascular surgery, most patients were treated by open repair (n=918, 88.6%), and only 11.4% of patients were treated by EVAR from January 2000 to July 2004, but the number of EVAR was increasing (10). Randomized controlled studies have conclusively demonstrated several initial benefits of EVAR compared to open repair in terms of early morbidity and mortality (4, 6). Vogel et al. (8) reported that EVAR groups showed excellent functional scores compared to open repair at the postoperative 3 months. Brewster et al. (9) reported that EVAR had increased rapidly, with 40-50% of all elective AAA patients in U.S.A., and at his institution, 65-70% of infrarenal AAA were recently treated by EVAR. Although early favorable results have broadened the applications of this treatment, the problems specific to EVAR, such as migration or dilation of endograft, endoleak, endotension, and device structural failure resulting in expansion or rupture of aneurysm, are documented in several mid-term results (5, 7, 9, 11, 17). Several reports described that inadequate patient selection for EVAR would cause a higher complication rate as well as long-term re-intervention rate (2, 15). However, stringent inclusion criteria may restrict the widespread adoption of this modern technology. Therefore, morphologic suitability of EVAR varies from 14% to 66%, possibly due to patient selection criteria, the methodology to measure anatomic factors, and the supplemental combination of multiple devices (11-13). Elkouri et al. (11) reported 14% of morphologic suitability with 2 currently available bifurcated endografts and the main reason for exclusion for EVAR was a poor proximal aortic neck. On the contrary, others reported a much higher rate of MS (12). They described that 66% of AAA patients were able to treat EVAR with 6 different types of endografts. They also found that inadequate proximal neck anatomy was the most common reason of exclusion for EVAR: a short proximal neck length (54%), inadequate access because of small iliac arteries (47%), wide neck (40%), bilateral CIA aneurysms extending to IIA (21%). To our knowledge, there were no reports that dealt with MS in Korea. In our study, 39% of MS is among the previously published rates of MS, and the main reason of exclusion for EVAR was the inadequate anatomy of proximal neck similar to other reports. There are some typical features in Asian people, such as a small diameter of Aorta and more frequent iliac aneurysm involvement and calcification. Owing to the anatomical difference of Koreans, such as relatively higher rates (EUROSTAR type C, D, E) (67.1%) of CIA aneurysm, however, the detailed proportions of exclusion in our study may be thought to be different from those of other ethnics. In the dream trial, there were type A in 32 (9.3%), type B in 215 (62.3%), type C in 36 (10.4%), type D in 29 (8.4%), and type E in 33 (9.6%). The CIA involvement rate with AAA was much lower (28.4%) compared with our study. With respect to anatomical features of AAA in Asians, Cheng et al. (18) reported a similar pattern to ours. They described that the CIA length was significantly shorter in Asians (right; 29.9 mm, left; 34.2 mm) than in Caucasians (p<0.001) and CIA diameter was wider (right; 20.2 mm, left; 17.9 mm). Those features might be resulted in one or both IIA exclusion more than 50% of patients. However, Masuda et al. (3) demonstrated that asian ethnics had smaller EIA diameters (p=0.0003) and more tortuous iliac arteries (p=0.03) but CIA diameter, maximal aneurysmal diameter, aortic neck diameter and iliac arterial calcification were not associated with statistically significant differences compared to non-asians in Hawaii. Therefore, it is not clear whether the anatomical difference of Korean may reduce MS of EVAR.
Welborn et al. (19) showed that the aneurysm size is intimately correlated with short neck, steep neck angulation, decreased CIA landing zones and more tortuosity, which should be decreased suitability of EVAR. Ouriel et al. (14) also described that as aneurysm size increased, several morphologic features including aneurysm length, angulation, and iliac artery size might be changed. Our study demonstrated consistent findings similar to above studies. Therefore, it is not surprising that the aneurysm size is a significant factor affecting complications, such as endoleaks or stent migration (2, 20, 21). Gender issues happen to be important in the patient selection for EVAR. Several investigators suggested that disproportionate numbers of women were excluded for EVAR owing to an access problem and small aorta size (8, 12, 17, 22). Additionally, some studies have shown that women had an increased length of hospital stay and need a post-discharge home care system following EVAR compared with men (8). However, their reason is not clearly examined. In our study, gender did not affect the feasibility of EVAR, which might be originated from the small numbers of women. The important prerequisite of clinical success of EVAR in extensive CIA aneurysm is IIA embolization. If CIA aneurysm extends to iliac bifurcation, one should conduct prophylactic iliac embolization to prevent endoleak from backflow of IIA and extend the endograft limb into EIA. However, if there is no blood supply from at least one of IIA, significant co-morbidity, namely pelvic ischemia, will be occurred. Pelvic ischemia can induce mostly buttock claudication, bowel ischemia, sexual dysfunction, and buttock claudication. Therefore, one should be cautious to keep at least one IIA open for bilateral CIA aneurysm, as many investigators have recommended (2, 12). There are several reports that tried to overcome the anatomical unsuitability. The treatment of poor proximal neck problem includes a combination of suprarenal fixation, fenestrated endograft and scallop, hook, or barbs (23, 24). To surmount poor distal fixation problems, several authors have described novel techniques, such as bell bottom technique, hypogastric bypass, or the use of bifurcated endograft into iliac bifurcation, to extend the application of EVAR for both iliac involvements (25, 26). If physicians who dealing with EVAR in Korea will generally adopt this policy, it will be more applicable with good functional outcomes.
In conclusion, when we follow the manufacturer's recommended criteria of 2 currently available endograft in Korea, 46.3% of MS will be feasible. If we stick to preserve at least one internal iliac artery to prevent pelvic ischemia, 39% of MS will be obtained in routine EVAR procedure. More efforts for fine skill and better understanding of stent graft design as well as patient anatomy will result in a higher feasibility in patients with AAA in Korea.
Figures and Tables
Fig. 2
Cases that were excluded for EVAR (A) CIA aneurysm involving both EIA and IIA. No feasible distal fixation zone was shown. (B) Inadequate infrarenal angle (125.9°). (C) Proximal short neck. Arrow indicates the lowest renal artery. The distance from lower margin of renal artery to aneurysm neck is about 5 mm. (D) Bilateral CIA aneurysm. Both CIA diameter was measured over 20 mm at the iliac bifurcation level.

References
1. Parodi JC, Palmaz JC, Barone HD. Transfemoral intraluminal graft implantation for abdominal aortic aneurysms. Ann Vasc Surg. 1991. 5:491–499.

2. Rockman C. Reducing complications by better case selection: anatomic considerations. Semin Vasc Surg. 2004. 17:298–306.

3. Masuda EM, Caps MT, Singh N, Yorita K, Schneider PA, Sato DT, Eklof B, Nelken NA, Kistner RL. Effect of ethnicity on access and device complications during endovascular aneurysm repair. J Vasc Surg. 2004. 40:24–29.

4. Greenhalgh RM, Brown LC, Kwong GP, Powell JT, Thompson SG. EVAR trial participants. Comparison of endovascular aneurysm repair with open repair in patients with abdominal aortic aneurysm (EVAR trial 1), 30-day operative mortality results: randomized controlled trial. Lancet. 2004. 364:843–848.
5. EVAR trial participants. Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomized controlled trial. Lancet. 2005. 365:2179–2186.
6. Prinssen M, Verhoeven EL, Buth J, Cuypers PW, Van Sambeek MR, Balm R, Buskens E, Grobbee DE, Blankensteijn JD; Dutch Randomized Endovascular Aneurysm Management (DREAM) Trial Group. A randomized trial comparing conventional and endovascular repair of abdominal aortic aneurysms. N Engl J Med. 2004. 351:1607–1618.

7. Drury D, Michaels JA, Jones L, Ayiku L. Systematic review of recent evidence for the safety and efficacy of elective endovascular repair in the management of infrarenal abdominal aortic aneurysm. Br J Surg. 2005. 92:937–946.

8. Vogel TR, Nackman GB, Crowley JG, Bueno MM, Banavage A, Odroniec K, Brevetti LS, Ciocca RG, Graham AM. Factors impacting functional health and resource utilization following abdominal aortic aneurysm repair by open and endovascular techniques. Ann Vasc Surg. 2005. 19:641–647.

9. Brewster DC, Jones JE, Chung TK, Lamuraglia GM, Kwolek CJ, Watkins MT, Hodgman TM, Cambria RP. Long-term outcomes after Endovascular abdominal aortic aneurysm repair. The first decade. Ann Surg. 2006. 244:426–438.
10. Kim YW, Min SK, Koh YB, Kim SN, Park JS, Moon IS, Park SW, Huh S, Choi JY, Park H, Cho WH, Kim HT, Park KH, Rhee JA, Cho KJ, Chung SW, Kim YS, Kim DI, Do YS, Kim SJ, Ha J, Park JH, Ahn H, Lee T, Choh JH, Kim D, Shim WH, Lee DY, Kwun KB, Suh BY, Kwun WH, Cho YP, Kim GE, Kwon TW, Cho HR, So BJ, Jun HJ, Kim SK, Chung SY, Choi SJ, Kim SH, Chang JH, Jang LC, Kim IG, Kim HC. Report of nation-wide Questionnaire survey for abdominal aortic aneurysm treatment in Korea. J Korean Vasc Surg. 2005. 21:10–15.
11. Elkouri S, Martelli E, Gloviczki P, McKusick MA, Panneton JM, Andrews JC, Noel AA, Bower TC, Sullivan TM, Rowland C, Hoskin TL, Cherry KJ. Most patients with abdominal aortic aneurysm are not suitable for endovascular repair using currently approved bifurcated stent-grafts. Vasc Endovascular Surg. 2004. 38:401–412.

12. Carpenter JP, Baum RA, Barker CF, Golden MA, Mitchell ME, Velazquez OC, Fairman RM. Impact of exclusion criteria on patient selection for endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2001. 34:1050–1054.

13. Cotroneo AR, Iezzi R, Giancristofaro D, Santoro M, Quinto F, Spigonardo F, Storto ML. Endovascular abdominal aortic aneurysm repair: how many patients are eligible for endovascular repair? Radiol Med (Torino). 2006. 111:597–606.

14. Ouriel K, Tanquilut E, Greenberg RK, Walker E. Aortoiliac morphologic correlations in aneurysms undergoing endovascular repair. J Vasc Surg. 2003. 38:323–328.

15. Green RM. Patient selection for endovascular abdominal aortic aneurysm repair. J Am Coll Surg. 2002. 194:Suppl 1. S67–S73.

16. Waasdorp EJ, De Vries JP, Hobo R, Leurs LJ, Buth J, Moll FL; EUROSTAR Collborators. Aneurysm diameter and proximal aortic neck diameter influnce clinical outcome of endovascular abdominal aortic repair; a 4-year EUROSTAR experience. Ann Vasc Surg. 2005. 19:755–761.
17. Aarts F, Van Sterkenburg S, Blankensteijn JD. Endovascular aneurysm repair versus open aneurysm repair: comparison of treatment outcome and procedure-related reintervention rate. Ann Vasc Surg. 2005. 19:699–704.

18. Cheng SW, Ting AC, Ho P, Poon JT. Aortic aneurysm morphology in Asians: features affecting stent-graft application and design. J Endovasc Ther. 2004. 11:605–612.
19. Welborn MB, Yau FS, Modrall JG, Lopez JA, Floyd S, Valentine RJ, Clagett GP. Endovascular repair of small abdominal aortic aneurysms: a paradigm shift? Vasc Endovascular Surg. 2005. 39:381–391.

20. Greenberg RK, Clair D, Srivastava S, Bhandari G, Turc A, Hampton J, Popa M, Green R, Ouriel K. Should patients with challenging anatomy be offered endovascular aneurysm repair? J Vasc Surg. 2003. 38:990–996.

21. Sampaio SM, Panneton JM, Mozes GI, Andrews JC, Bower TC, Karla M, Noel AA, Cherry KJ, Sullivan T, Gloviczki P. Proximal type I endoleak after endovascular abdominal aortic aneurysm repair: predictive factors. Ann Vasc Surg. 2004. 18:621–628.

22. Moise MA, Woo EY, Velazquez OC, Fairman RM, Golden MA, Mitchell ME, Carpenter JP. Barriers to endovascular aortic aneurysm repair: past experience and implications for future device development. Vasc Endovascular Surg. 2006. 40:197–203.

23. Noel AA. Beyond the instructions for use:pushing the limits of infrarenal device application for abdominal aortic aneurysms. Perspect Vasc Surg Endovasc Ther. 2006. 18:19–23.
24. Verhoeven EL, Prins TR, Tielliu IF, van den Dungen JJ, Zeebregts CJ, Hulsebos RG, van Andringa de Kempenaer MG, Oudkerk M, van Schilfgaarde R. Treatment of short-necked infrarenal aortic aneurysm with fenestrated stent-graft: short-term results. Eur J Vasc Endovasc Surg. 2004. 27:477–483.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download