Abstract
Bacille Calmette-Guérin (BCG) induces potent Th1 responses with the help of interleukin (IL)-10 and IL-12 released from dendritic cells (DCs), and suppresses Th2-associated allergic reactions. However, there are still some controversies on therapeutic effects of BCG in asthmatics. This study investigated whether BCG administration to DCs suppresses IL-5 production from T cells in atopic asthmatics. DCs derived from peripheral blood of subjects were cultured with or without BCG and Dermatophagoides farinae extract. Some DCs were co-cultured with T cells in the presence of BCG or the above culture supernatants. In the atopic asthmatics, BCG significantly increased IL-10 and IL-12 production from DCs. In the presence of D. farinae extract, BCG further increased IL-10 production. BCG-induced IL-10 production was significantly higher in the atopics (n=14) than in the non-atopics (n=9). Both BCG and the BCG-treated DCs culture supernatant significantly increased IFN-γ production from T cells. Both BCG and the supernatant from DCs+BCG+D. farinae co-cultures significantly decreased IL-5 production (all p<0.05), but the supernatant from DCs+BCG co-cultures did not. In conclusion, administration of BCG together with D. farinae extract effectively decreased IL-5 production from T cells, probably through the action of IL-10 and IL-12 released from DCs in D. farinae-sensitive asthmatics.
The 'hygiene hypothesis' states that advancing hygiene eliminates environmental cues, such as infections, that skew adaptive immunity away from Th2 responses and causes a striking increase in allergic diseases including asthma (1). In this context, many animal studies have shown that bacille Calmette-Guérin (BCG) and other mycobacterial agents suppress Th2-associated allergy and asthma, probably through Th1 (2-5) or regulatory T cell (6) immune responses. In addition, we previously demonstrated that BCG vaccination improves lung function and reduces medication use in adult patients with asthma (7), while other investigators failed to show any beneficial effect (8, 9) or showed only a limited effect (10). This discrepancy in the effects of BCG vaccination maybe related to the factors affecting the efficacy including timing of vaccination, the route of delivery, genetics and ethnicity, exposure to environmental mycobacteria (11), and BCG strain (12).
The interaction of BCG and toll-like receptor (TLR) 2 on dendritic cells (DCs) may affect the differentiation of naïve T cells mainly to Th1-like cells with the help of interleukin (IL)-10, IL-12, and so forth (11). However, in addition to the above controversies on the therapeutic effect of BCG in patients with asthma, De Wit et al. (13) showed that when DCs from atopic patients were pulsed beforehand with an allergen, IL-12 could not inhibit IL-5 production by T cells. Because co-administration of ovalbumin with IL-18 (14) or CpG motifs (15) effectively suppresses production of Th2 cytokines, we can speculate that simultaneous administration of BCG with an allergen may suppress Th2 responses even though BCG alone is not so much effective in established allergic diseases.
To support our previous report (7) shown beneficial effects of BCG on human asthma and to elucidate the underling mechanism for the effects, we observed the responses to BCG of DCs and T cells from asthma patients sensitized to Dermatophagoides farinae (D. farinae) with exposing both allergen and BCG simultaneously in this study.
A study that examined the relationship between dyspnea perception and airway hyper-responsiveness to methacholine in asthma was conducted at Chonnam National University Hospital, Gwangju, Korea, between October and December 2005. The study enrolled medical students, hospital employees including doctors and nurses, and patients who required a medical certificate for asthma in order to be exempted from obligatory military service, mostly young adult men. Forty of the 63 adult volunteers who participated in that study donated their blood (50 mL) for the present study. However, two of them did not meet the criteria for group classification in this study, and appropriate numbers of DCs were harvested from the blood samples of 23 of the remaining 38 volunteers. The data from the 23 subjects were used for the statistical analysis, and clinical characteristics of subjects are presented in Table 1.
The group criteria were as follows: non-atopic normal group, healthy subjects who were not sensitized to ten common aeroallergens; atopic normal group, healthy subjects sensitized to D. farinae; non-atopic asthma group, asthma patients who were not sensitized to ten common aeroallergens; and atopic asthma group, asthma patients sensitized to D. farinae. Before the study, all of the subjects withheld their medications for asthma or allergy for one week and bronchodilators for 12 hr. The Institutional Review Board of Chonnam National University Hospital approved this study. All of the subjects were informed of the experimental procedures, and all provided written informed consent.
On the study day, allergy skin-prick tests and methacholine bronchoprovocation test were performed after taking a history and conducting a physical examination. Then, if the above criteria were met, laboratory tests for complete blood counts and serum total IgE and blood sampling for peripheral blood mononuclear cell culture were performed. DCs were isolated from the blood cells and cultured with or without BCG and D. farinae extract. Some DCs were co-cultured with T cells in the presence of BCG or the above culture supernatants, and T cell proliferation and cytokine levels were measured.
Allergy skin-prick tests were conducted using ten common aeroallergen extracts (Allergopharma, Reinbek, Germany): D. farinae, Dermatophagoides pteronyssinus, cockroach, cat, dog, Aspergillus, hazel, birch, timothy, and ragweed. Histamine (1 mg/mL) and saline (0.9%) solutions were used as the positive and negative controls, respectively. Skin test reactivity was graded according to the ratio of the size of the allergen-induced wheal to the size of the wheal elicited by the histamine solution, and was categorized as follows: 1+: 25-49%, 2+: 50-99%, 3+: 100-199%, 4+: ≥200%. The sum of the grades for the ten allergens was defined as 'atopy score', and the reactivity of ≥3+ or <1+ was regarded as indicating clinically significant positive or negative responses, respectively. The bronchial challenge tests followed a standardized tidal breathing method (16). A positive test response was defined as a 20% fall in the forced expiratory volume in 1 sec in response to a provocation concentration of methacholine of <16 mg/mL. Asthma was defined as asthma symptoms and a positive bronchial challenge response.
Dendritic cells were generated from peripheral blood mononuclear cells (PBMCs), as described by Romani et al. (17). Briefly, PBMCs from the volunteers were isolated by density gradient centrifugation using Lymphoprep (Nycomed, Oslo, Norway). The CD14+ cells were isolated from the PBMCs using an LS-MACS column (Miltenyi Biotec, Auburn, CA, U.S.A.), in which the purity of the CD14+ cells exceeded 95%. DCs were generated by culturing the CD14+ cells (1 × 106/mL) with 50 ng/mL GM-CSF (LG Biochemical, Daejeon, Korea) and 50 ng/mL IL-4 (R & D Systems, Minneapolis, MN, U.S.A.) for 6 days in T-25 culture flasks (Nunc, Roskilde, Denmark) containing RPMI 1640 (BioWhittaker, Walkersville, MD, U.S.A.) supplemented with 10% fetal bovine serum (Gibco, Grand Island, NY, U.S.A.), 100 U/mL penicillin, 100 µg/mL streptomycin, and 0.25 µg/mL amphotericin B (BioWhittaker). CD3+ T cells were isolated using human T cell enrichment columns (R & D Systems), in which the purity of the CD3+ cells exceeded 90%, and the T cells were stored in liquid nitrogen until culture.
Live attenuated BCG (Tokyo 172 strain; Korean National Tuberculosis Association, Seoul) was cultured for 3 weeks in a semisolid mixture of Middlebrook 7H10 agar (Becton Dickinson, Sparks, MD, U.S.A.) and Middlebrook 7H9 broth (Becton Dickinson) supplemented with 10% Middlebrook oleic acid-albumin-dextrose catalase (Becton Dickinson). The colonies were counted before preparing the inoculum. DCs (1 × 105/mL) were cultured with 1.5 × 105 colony-forming units (CFUs) of BCG with or without D. farinae extract (group 1 allergen, 10 µg/mL; Allergy Research Institute of Yonsei University, Seoul) for 24 hr. The culture supernatants were used for IL-10 and IL-12 assays or were added to the DC-T cell co-cultures described below.
CD3+ T cells were thawed on the day of use. T cells (1 × 105/mL) were co-cultured with irradiated (30 Gy) autologous DCs at a 10:1 ratio for 4 days. BCG (1.5 × 105 CFUs) or the culture supernatants from BCG-treated DCs (100 µL/well) together with D. farinae extract were added at the beginning of the DC-T cell co-culture. The supernatants were collected from the cultures on day 3 and stored at -70℃ for cytokine assays of IFN-γ and IL-5.
T cell proliferation during the last 18 hr of 4-day cultures was quantified using [3H] thymidine uptake of cells incubated with 1 µCi of [methyl-3H]-thymidine (Amersham Pharmacia Biotech, Buckinghamshire, U.K.). The cells were harvested onto paper filters using a Titertek Cell Harvester 530 (Flow Laboratories, Irvine, U.K.), and the radioactivity was measured using a Beckman LS 6500 multipurpose scintillation counter (Beckman Instruments, Fullerton, CA, U.S.A.). The results are presented as the mean counts per minute (cpm) of triplicated cultures. The concentrations of IL-5, IL-10, IL-12, and IFN-γ in the culture supernatants were determined using commercial enzyme-linked immunosorbent assay (ELISA) kits (BioSource, Nivelles, Belgium). The sensitivities of the assays for IL-5, IL-10, IL-12, and IFN-γ were 4, 1, 2, and 4 pg/mL, respectively
The data were expressed as the mean±SEM. To determine the significance of inter-group and intra-group differences, the chi-square test, Kruskal-Wallis test, Mann-Whitney U test and Wilcoxon's signed-rank test were used. A value of p<0.05 was considered statistically significant.
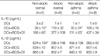
In the atopic asthma group, BCG treatment significantly increased IL-10 and IL-12 productions by DCs. In the presence of D. farinae extract, BCG further increased the production of IL-10 but not IL-12 (Fig. 1). The other study groups also showed a similar trend (Table 2). In addition, the IL-10 level in the culture supernatants of DCs+BCG with (p<0.01) or without (p<0.05) D. farinae were significantly higher in the atopic asthma group (n=8) than in the non-atopic asthma group (n=5) and than in the non-atopic normal group (n=4, p<0.05). The atopic normal group showed a similar trend with the atopic asthma group, and so BCG-induced IL-10 productions were significantly higher in the atopics (n=14) than in the non-atopics (n=9) (all p<0.01). The baseline IL-12 level was significantly lower in the atopic asthma group than in the non-atopic normal group (p<0.05). The atopic normal group showed a similar trend with the atopic asthma group, and so the baseline IL-12 level was significantly lower in the atopics than in the non-atopics (p<0.05).
In the atopic asthma group, BCG treatment significantly increased T cell proliferation (Fig. 2). And the D. farinae extract alone increased T cell proliferation. However, the addition of the culture supernatants from DCs+BCG co-cultures resulted in a significantly lower proliferation index (3H-thymidine incorporation) than BCG did. The other study groups also showed a similar trend without statistical significance.
Similarly, IFN-γ production was increased significantly with BCG treatment in the atopic asthma group (Fig. 3). BCG tended to further increase IFN-γ production in the presence of D. farinae extract (p=0.13). The supernatants from DCs+BCG co-cultures also significantly increased IFN-γ production. However, as compared with BCG, the supernatant from DCs+BCG cultured in the absence of D. farinae extract resulted in significantly lower IFN-γ production (p<0.05). Similar trends were observed for the other study groups without statistical significance (Table 3). In addition, the IFN-γ levels were significantly lower in the non-atopic asthma group (n=4) in the most comparisons as compared with the non-atopic normal group (n=4). The atopic asthma group showed a similar trend with the non-atopic asthma group, and the baseline and BCG+D. farinae IFN-γ levels were significantly lower in the asthmatics than in the non-asthmatics (all p<0.05).
In the atopic asthma group, D. farinae extract significantly increased IL-5 production in DCs-T cell co-cultures (Fig. 4). Both BCG and the culture supernatant from DCs+BCG+D. farinae co-cultures significantly decreased IL-5 production. However, the culture supernatants from DCs+BCG co-cultures did not significantly decrease IL-5 production. Similarly, D. farinae extract tended to increase IL-5 production in the other study groups also, but neither BCG nor the culture supernatant showed a suppressive effect on IL-5 production (Table 3). The IL-5 levels in the DCs-T+BCG co-culture supernatants tended to be lower in the atopic normal (p=0.08) and atopic asthma (p=0.06) groups than in the non-atopic normal group.
In this study, BCG infection of DCs and T cell co-cultures increased IFN-γ and decreased IL-5 production from T cells in atopic asthmatics. These results are consistent with previous reports (2-7) that BCG has suppressive effects on asthma. This study extended our previous report (18) showing that the cytokine milieu secreted by BCG-treated DCs directly enhanced IFN-γ production from T cells. The DCs+BCG culture supernatant used in the previous study (18) did not significantly suppress IL-5 production in the present study too, but the DCs+BCG+D. farinae culture supernatant as well as BCG did.
In addition, we found that BCG further increased IL-10 production from DCs in the presence of D. farinae extract. This adjuvant effect of D. farinae extract was also observed in increasing IFN-γ production and in suppressing IL-5 production from T cells. These phenomena may be explained, at least in part, by more efficient polarization by simultaneous exposure of BCG and D. farinae to DCs. Shirota et al. (15) demonstrated that low-dose CpG alone had no effect on eosinophilia but had an efficient anti-allergic effect upon co-inoculation with ovalbumin. Moreover, Kim et al. (14) showed that the fusion of ovalbumin with IL-18 was more efficient than a simple mixture of ovalbumin and IL-18 in inducing IFN-γ production. When DCs present allergens to T cells, a 'signal 3' such as IL-12, which is induced through TLRs by microbes (including BCG) and other factors, determines cell polarization (19). In this process, manipulation by the same or neighboring DCs of both an allergen and BCG administered together may more efficiently induce the allergen-specific Th1 immune response (20). Although the adjuvant effect of D. farinae extract on IFN-γ production was associated with increase in IL-10 production, the effect might be caused not by the inhibitory cytokine IL-10, but by IL-2 and IFN-α that are known to be related with BCG-induced IFN-γ production (21). And, the potent BCG might induce maximal production of IL-12 without remaining a space for increase by D. farinae extract. However, further investigation is needed in these regards.
In the present study, IL-10 and IL-12 productions from DCs were increased in response to BCG, and the DCs+BCG+D. farinae culture supernatant containing these cytokines increased IFN-γ and decreased IL-5 production from T cells. De Wit et al. (13) showed that IL-5 production by T cells from atopic individuals was not inhibited in the presence of exogenous recombinant IL-12, the best-studied Th1-driving factor (18). The timing of the allergen exposure to DCs might be related to this discrepancy between the two studies. De Wit et al. (13) cultured T cells with DCs pulsed beforehand with D. pteronyssinus, in contrast to our DCs-T+D. farinae co-culture. Although chronic antigen stimulation has been found to render the T-cell (22) and DC (23) subsets more resistant to change, reversals have been shown to occur in animals (3, 5) and humans (7, 24). Thus, our results suggest that simultaneous exposure of Th1 polarizing signal with an allergen may suppress Th2 responses even in established allergic diseases.
In addition, the DCs+BCG±D. farinae culture supernatants, i.e., a cytokine milieu containing IL-10 and IL-12, were effective, but less so than BCG per se, in inducing Th1 polarization in atopic asthmatics in this study. BCG, but not the DCs+BCG culture supernatant, induced T cell proliferation over D. farinae alone did. Therefore, BCG-induced T cell proliferation might be related, at least in part, to the differential increase in Th1 polarizing activity over the cytokine milieu-induced signal 3. For a productive immune response, co-stimulatory signals (signal 2) as well as signal 3 induced by BCG must work together.
Compared with non-atopic individuals, atopic subjects produced more IL-10 from DCs and less IL-5 from T cells in response to BCG in this study. T regulatory 1 (Tr1) cells, which are induced by IL-10 (25), would produce high levels of IL-10 and suppress IL-5 production (26). Th1 as well as Th2 cells are suppressed by Tr1 cells, but the power of IL-12 inducing Th1 cells may overcome the effect of Tr1 cells resulting in an increase in IFN-γ production from T cells. In contrast to our results, Gentile et al. (27) demonstrated that lipopolysaccharide-induced IL-10 production from DCs was diminished in patients with asthma and allergic rhinitis, and suggested that atopic subjects may have an intrinsic inability to up-regulate DC IL-10 production. Diminished production of IL-12 also has been suggested as a risk factor for atopy (28), as our result showing that the baseline IL-12 production from DCs was significantly lower in the atopic asthma group than in the non-atopic normal. However, even if atopic individuals are prone to develop allergic diseases due to low levels of the baseline IL-10 and IL-12, the potent Th1/Tr1 inducer BCG may overcome this barrier easily. BCG efficiently and rather more remarkably increased production of both IL-10 and IL-12 from DCs in the atopics than in the non-atopics in this study.
In the non-atopic asthmatics, BCG induced significantly lower IL-10 production than in the atopic asthmatics and lower IFN-γ production than in non-atopic normal individuals. Although it has been suggested that low IFN-γ levels are a characteristic of asthma, regardless of the atopic state (29), it should be determined whether BCG vaccination is less effective in suppressing non-atopic asthma than atopic asthma. Interestingly, the atopic normals did not show a higher production of IL-5 in response to D. farinae extract than the non-atopics did. It remains to be determined whether sensitized but not allergic subjects have any mechanism that controls allergen-induced IL-5 production and eosinophilia. This study has some limitations. First of all, the number of study subjects was too small. Secondly, we are not sure that the D. farinae extract was not contaminated with lipopolysaccharide. The D. farinae extract augmented BCG-induced IL-10 production from DCs even in the non-atopic individuals in this study. In these regards, further studies are needed.
In conclusion, administration of BCG together with D. farinae extract effectively decreased IL-5 production from T cells, probably through the action of IL-10 and IL-12 released from DCs in D. farinae-sensitive asthmatics.
Figures and Tables
Fig. 1
Interleukin (IL)-10 and IL-12 concentrations in the supernatant of dendritic cell (DC) culture with or without BCG and Dermatophagoides farinae (Df) extract in the atopic asthmatics (n=8).
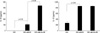
Fig. 2
T cell proliferation in the supernatant of co-cultures of dendritic cells and T cells from the atopic asthmatics (n=8) with or without BCG, Dermatophagoides farinae (Df) extract and supernatant of dendritic cells-BCG co-culture. Sup1, supernatant of dendritic cells and BCG co-culture; Sup2, supernatant of dendritic cells, BCG, and Df co-culture.

Fig. 3
Interferon (IFN)-γ concentration in the supernatant of co-cultures of dendritic cells and T cells from the atopic asthmatics (n=8) with or without BCG, Dermatophagoides farinae (Df) extract and supernatant of dendritic cells-BCG co-culture. Sup1, supernatant of dendritic cells and BCG co-culture; Sup2, supernatant of dendritic cells, BCG, and Df co-culture.

Fig. 4
Interleukin (IL)-5 concentration in the supernatant of dendritic cell and T cell co-cultures from the atopic asthmatics (n=8) with or without BCG, Dermatophagoides farinae (Df) extract and supernatant of dendritic cells-BCG co-culture. Sup1, supernatant of dendritic cells and BCG co-culture; Sup2, supernatant of dendritic cells, BCG, and Df co-culture.

Table 2
Comparisons of Interleukin (IL)-10 and IL-12 concentrations in the culture supernatants of dendritic cells (DCs) among study groups
References
1. Cameron L, Vercelli D. Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FER, editors. Synthesis and regulation of immunoglobulin E. Middleton's allergy principles & practice. 2003. 6th ed. Philadelphia: Mosby Inc.;87–100.
2. Herz U, Gerhold K, Gruber C, Braun A, Wahn U, Renz H, Paul K. BCG infection suppresses allergic sensitization and development of increased airway reactivity in an animal model. J Allergy Clin Immunol. 1998. 102:867–874.

3. Erb KJ, Holloway JW, Sobeck A, Moll H, Le Gros G. Infection of mice with Mycobacterium bovis-Bacillus Calmette-Guerin (BCG) suppresses allergen-induced airway eosinophilia. J Exp Med. 1998. 187:561–569.
4. Wang CC, Rook GA. Inhibition of an established allergic response to ovalbumin in BALB/c mice by killed Mycobacterium vaccae. Immunology. 1998. 93:307–313.

5. Hopfenspirger MT, Agrawal DK. Airway hyperresponsiveness, late allergic response, and eosinophilia are reversed with mycobacterial antigens in ovalbumin-presensitized mice. J Immunol. 2002. 168:2516–2522.

6. Zuany-Amorim C, Sawicka E, Manlius C, Le Moine A, Brunet LR, Kemeny DM, Bowen G, Rook G, Walker C. Suppression of airway eosinophilia by killed Mycobacterium vaccae-induced allergen-specific regulatory T-cells. Nat Med. 2002. 8:625–629.

7. Choi IS, Koh YI. Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol. 2002. 88:584–591.

8. Shirtcliffe PM, Easthope SE, Cheng S, Weatherall M, Tan PL, Le Gros G, Beasley R. The effect of delipidated deglycolipidated (DDMV) and heat-killed Mycobacterium vaccae in asthma. Am J Respir Crit Care Med. 2001. 163:1410–1414.
9. Shirtcliffe PM, Easthope SE, Weatherall M, Beasley R. Effect of repeated intradermal injections of heat-inactivated Mycobacterium bovis bacillus Calmette-Guerin in adult asthma. Clin Exp Allergy. 2004. 34:207–212.

10. Vargas MH, Bernal-Alcantara DA, Vaca MA, Franco-Marina F, Lascurain R. Effect of BCG vaccination in asthmatic schoolchildren. Pediatr Allergy Immunol. 2004. 15:415–420.

11. Barlan I, Bahceciler NN, Akdis M, Akdis CA. Bacillus Calmette-Guerin, Mycobacterium bovis, as an immunomodulator in atopic diseases. Immunol Allergy Clin North Am. 2006. 26:365–377.

12. Choi IS, Lin XH, Koh YA, Koh YI, Lee HC. Strain-dependent suppressive effects of BCG vaccination on asthmatic reactions in BALB/c mice. Ann Allergy Asthma Immunol. 2005. 95:571–578.

13. De Wit D, Amraoui Z, Vincart B, Michel O, Michils A, Van Overvelt L, Willems F, Goldman M. Helper T-cell responses elicited by Der p 1-pulsed dendritic cells and recombinant IL-12 in atopic and healthy subjects. J Allergy Clin Immunol. 2000. 105:346–352.

14. Kim SH, Cho D, Hwang SY, Kim TS. Efficient induction of antigen-specific, T helper type 1-mediated immune responses by intramuscular injection with ovalbumin/interleukin-18 fusion DNA. Vaccine. 2001. 19:4107–4114.

15. Shirota H, Sano K, Kikuchi T, Tamura G, Shirato K. Regulation of T-helper type 2 cell and airway eosinophilia by transmucosal coadministration of antigen and oligodeoxynucleotides containing CpG motifs. Am J Respir Cell Mol Biol. 2000. 22:176–182.

16. Sterk PJ, Fabbri LM, Quanjer PH, Cockcroft DW, O'Byrne PM, Anderson SD, Juniper EF, Malo JL. Airway responsiveness. Standardized challenge testing with pharmacological, physical and sensitizing stimuli in adults. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. Eur Respir J Suppl. 1993. 16:53–83.
17. Romani N, Gruner S, Brang D, Kampgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch PO, Steinman RM, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994. 180:83–93.

18. Koh YI, Choi IS, Lee JJ. Effects of cytokine milieu secreted by BCG-treated dendritic cells on allergen-specific Th immune response. J Korean Med Sci. 2004. 19:640–646.

19. Kapsenberg ML, Jansen HM. Adkinson NF, Yunginger JW, Busse WW, Bochner BS, Holgate ST, Simons FE, editors. Antigen presentation and immunoregulation. Middleton's allergy principles & practice. 2003. Philadelphia: Mosby;177–188.
20. Sano K, Haneda K, Tamura G, Shirato K. Ovalbumin (OVA) and Mycobacterium tuberculosis bacilli cooperatively polarize anti-OVA T-helper (Th) cells toward a Th1-dominant phenotype and ameliorate murine tracheal eosinophilia. Am J Respir Cell Mol Biol. 1999. 20:1260–1267.
21. Chen X, O'Donnell MA, Luo Y. Dose-dependent synergy of Th1-stimulating cytokines on bacilli Calmette-Guérin-induced interferongamma production by human mononuclear cells. Clin Exp Immunol. 2007. 149:178–185.
22. Murphy E, Shibuya K, Hosken N, Openshaw P, Maino V, Davis K, Murphy K, O'Garra A. Reversibility of T helper 1 and 2 populations is lost after long-term stimulation. J Exp Med. 1996. 183:901–913.

23. Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000. 164:4507–4512.

24. Arkwright PD, David TJ. Intradermal administration of a killed Mycobacterium vaccae suspension (SRL 172) is associated with improvement in atopic dermatitis in children with moderate-to-severe disease. J Allergy Clin Immunol. 2001. 107:531–534.

25. Groux H, O'Garra A, Bigler M, Rouleau M, Antonenko S, de Vries JE, Roncarolo MG. A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature. 1997. 389:737–742.
26. Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001. 182:68–79.

27. Gentile DA, Schreiber R, Howe-Adams J, Trecki J, Patel A, Angelini B, Skoner DP. Diminished dendritic cell interleukin 10 production in atopic children. Ann Allergy Asthma Immunol. 2004. 92:538–544.

28. Reider N, Reider D, Ebner S, Holzmann S, Herold M, Fritsch P, Romani N. Dendritic cells contribute to the development of atopy by an insufficiency in IL-12 production. J Allergy Clin Immunol. 2002. 109:89–95.

29. Oliveira FH, Sarinho SW, Montenegro S, Neuenschwander C, Queiroz R, Medeiros D, Schor D, Sarinho E. Production of interferon gamma in asthmatic patients with small bacille Calmette-Guerin scars: a pilot study. Allergy Asthma Proc. 2006. 27:516–522.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download