Abstract
The understanding of main mechanisms that determine the ability of immune privilege related to Sertoli cells (SCs) will provide clues for promoting a local tolerogenic environment. In this study, we evaluated the property of humoral and cellular immune response modulation provided by porcine SCs. Porcine SCs were resistant to human antibody and complement-mediated formation of the membrane attack complex (38.41±2.77% vs. 55.02±5.44%, p=0.027) and cell lysis (42.95±1.75% vs. 87.99±2.25%, p<0.001) compared to immortalized aortic endothelial cells, suggesting that porcine SCs are able to escape cellular lysis associated with complement activation by producing one or more immunoprotective factors that may be capable of inhibiting membrane attack complex formation. On the other hand, porcine SCs and their culture supernatant suppressed the up-regulation of CD40 expression (p<0.05) on DCs in the presence of LPS stimulation. These novel findings, as we know, suggest that immune modulatory effects of porcine SCs in the presence of other antigen can be obtained from the first step of antigen presentation. These might open optimistic perspectives for the use of porcine SCs in tolerance induction eliminating the need for chronic immunosuppressive drugs.
Neonatal porcine Sertoli cells (NPSCs) are known to survive long-term as discordant xenografts in non-immunosuppressed rodents (1). It was previously shown that Setoli cells (SCs) express complement regulatory proteins (CRPs), which may exert their effect on the humoral-mediated immune response (2, 3). Identification of CRPs in SCs suggests that they can survive complement-mediated cytolysis by producing a combination of complement inhibitors, resulting in protection against destruction by complement activation. Consistent with these observations, SCs are also known to produce high levels of clusterin, which has been shown to inhibit formation of the membrane attack complex (MAC) in vitro (4). These characteristics of SCs have raised the possibility that they can be used as candidates for cellular therapy or can be used as vehicles. However, the expression of CRPs in NPSCs was evaluated only in terms of mRNA levels, and the relative efficiency of CRPs from one species at regulating complement from a distant species remains a point of controversy (5, 6).
It is known that SCs exert the local immune environment via a paracrine interaction with immune cells. They are known to produce TGFβ, which is suspected to have anti-inflammatory properties and is required for an immune-privileged microenvironment. In fact, TGFβ from SCs modulates cell activity and favors Th2 over Th1 cell differentiation, as indicated by the phenotype of the infiltrating T cells in a manner that is dependent on the secretion of TGFβ(7).
SCs are known to protected themselves from cell-mediated rejection when transplanted as allografts and xenografts (8, 9). Additionally, they were able to prevent the rejection of other cells (10). Although various mechanisms to explain the local immune-suppressive effect of SCs are under consideration, it remains to be determined whether or how SCs would modulate a cell-mediated immune response that resulted in the induction of systemic tolerance. We can hypothesize that immune modulatory mechanisms of SCs can be obtained from the first step of antigen presentation. The induction of the cellular immune response following transplantation involves a complex interaction between antigen presenting cells (APCs) and T cells. Dendritic cells (DCs), known to be the most potent of the APCs, play a key role in directing cellular and humoral immune responses against self and non-self antigens (11). DCs present endogenously-expressed antigens as well as exogenous antigens acquired through endocytic and phagocytic pathways (12). Conversely, DCs can exhibit tolerogenic properties, which seem to depend directly on their functional state of maturation and degree of activation (13). In fact, it has been shown that immature DCs (iDCs) may induce T cell hyporesponsiveness or regulatory T (Tr) cells (14). This suggests that a strategy to prevent full activation of DCs during primary sensitization may be more effective than conventional immunosuppressive agents that broadly inhibit the activation of T lymphocytes.
In this study, we tested whether porcine SCs can survive as xenografts against human antibody- and complement-mediated humoral immune responses in vitro. Also, we tried to elucidate the effect of porcine SCs on the activation and maturation of DCs in the presence of antigenic stimuli.
C57BL/6 male mice, aged 6 weeks, were purchased form Orient Corporation (Seongnam, Korea) and used as bone marrow donor at the age of 7-9 weeks. All animal experiments were performed with approval of the Institutional Animal Care and Use Committee (IACUC) of Clinical Research Institute in Seoul National University Hospital. And National Research Council (NRC) 'Guidelines for the Care and Use of Laboratory Animals' were observed (revised 1996).
Immortalized porcine SC line (NPSCi), was created by transfection of SV40 large T antigen to NPSCs as previously reported (3). Immortalized porcine aortic endothelial cell line (PECi) was a gift from professor Curie Ahn (Seoul National University, College of Medicine, Seoul, Korea). They were maintained in 1:1 mixture of Ham's F12/DMEM containing 5% fetal bovine serum (FBS, Gibco Laboratories, Grand Island, NY, U.S.A.) supplemented with 10 mM HEPES, 5 µg/mL insulin (Sigma-Aldrich Inc., St. Louis, MO, U.S.A.), 10 µg/mL transferrin (Sigma-Aldrich Inc.), 10 µg/mL vitamin E (Sigma-Aldrich Inc.), 4 µg/mL hydrocortisone (Sigma-Aldrich Inc.), and antimycotic/antibiotics (Gibco Laboratories) at 37℃.
Both cell lines, cultured overnight on chamber slides, were fixed with absolute cold ethanol for 2 hr and stained for Griffonia simplicifolia isolectin B4 (GS-IB4, Molecular Probes Inc., OR, U.S.A., 1:100). As a control for nonspecific binding, cells were also stained as described above without IB4. To conform the results of immunohistochemistry that are positive for Gal, 1×106 non-fixed single cells that had been cultured for 24 hr were incubated with biotin-conjugated GS-IB4 (1:200) for 30 min on ice, washing twice, fluorescein isothiocyanate-conjugated streptavidin (FITC-SA, 1:500), and analyzed by flow cytometry (FACS).
To determine the binding of preformed human xenoreactive antibodies to cell lines, 1×106 non-fixed single cells after culture for 48 hr were incubated with heat-inactivated human O serum (iNHS, 50% in staining buffer) for 1 hr at 37℃, followed by monoclonal mouse anti-human IgM (DAKO Diagnostics, Carpinteria, CA, U.S.A., 1:50), and FITC-conjugated rat anti-mouse IgG (Jackson Immuno Research Lab, Inc., PA, U.S.A., 1:500) for 30 min on ice. Negative controls were consisted of cells that were incubated in the absence of human serum. The percentage of cells bound by anti-human antibodies was determined using FACS analysis.
To determine the binding of preformed xenoreactive antibodies to both cell lines, 1×106 non-fixed single cells after culture for 48 hr were incubated with freshly prepared normal human O serum (NHS, 50% in staining buffer) for 1 hr at 37℃, followed by monoclonal mouse anti-human C5b-9 (DAKO Diagnostics, 1:50) and FITC-conjugated rat anti-mouse IgG (Jackson ImmunoResearch Lab, Inc., 1:500) for 30 min on ice. Negative controls consisted of cells that were incubated without human serum. The percentage of cells bound by human antibodies was determined by FACS analysis.
The in vitro human antibody- and complement-mediated cytotoxicity assay was performed similar to that described previously (15). Briefly, 4×104 cells were plated in 48-well tissue culture plates and cultured in 0.5 mL of 1:1 mixture of Ham's F12/DMEM (supplemented as above). After 48 hr, 0.1 mL (final 20%) of media were replaced with fresh media (M), fresh media plus 50 µL of 3-4 week-old rabbit complement (M+C, Cedarlane Laboratories Limited, NC, U.S.A.), iNHS or NHS. Cells were additionally incubated at 37℃ for 1 or 4 hr. Each well was washed twice with culture media, and replaced 0.5 mL fresh culture media. For evaluation of cytotoxicity, MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide, Sigma-Aldrich Inc.) assay were performed. Briefly, 50 µL MTT stock (1 mg/mL in PBS) were added to each well. After incubation of 4 hr at 37℃ in humidified CO2 incubator, aspirate the medium containing MTT, and then add 150 µL of DMSO to dissolve MTT-formazan crystals. The absorbance of the supernatant was measured at 570 nm using a spectrophotometer with a reference wavelength of 650 nm. All assays were performed in triplicate with data expressed as mean absorbance.
Monocytes from bone marrow were differentiated into DCs in the presence of granulocyte monocyte colony stimulating factor (GM-CSF) containing culture medium. Briefly, bone marrow cell suspensions were isolated from femurs of C57BL/6 mice and propagated in 12-well plates (5×105 cells/well) in 2 mL RPMI-1640 medium containing antibiotics, 10% FBS and low-dose GM-CSF (3.5 ng/mL). The culture medium was changed every 2 days. On day 6, loosely adherent cells were used as resting/immature DCs. Combination of dexamethasone (Dexa, 10-6 M, Sigma-Aldrich Inc.) and active form of vitamin D3, 1,25(OH)2D3 (D3, 10-10 M, Sigma-Aldrich Inc.) were added on day 2 and 4 to inhibit maturation of DCs as reported previously (16) as control. The DCs as iDCs were tested for surface molecule expressions by FACS analysis.
The effects of porcine SCs and their culture supernatant (iSPNT, prepared by 48 hr culture of immortalized porcine SCs at a density of 5×105 cell/10 mL culture media) on DCs during activation and maturation were investigated. Briefly, 1×106 iDCs were co-cultured for 24 hr with different ratio of NPSCi (2×105 or 5×105 in total 3 mL) or iSPNT (1 mL in total 2 mL) in the presence or absence of lipopolysaccharide (LPS, 1 µg/mL) stimulation.
Cells were stained with FITC- or Phycoerythrin (PE)-conjugated rat anti-mouse monoclonal antibodies (BD Pharmingen, San Diego, CA, U.S.A.) for CD40 (Cat No. 553723), CD80 (Cat No. 553768), and MHC II (I-Ab, Cat No. 553551), or CD11c (Cat No. 553802) after blocking nonspecific binding with 2.4G2.
Data were expressed as mean±SD of n independent experiments. The statistical significance of difference between multiple comparisons was calculated by one-way analysis of variance (ANOVA). The Scheffé F-test was used to determine specific differences between means when determined as significant by ANOVA. A p value of <0.05 was considered significant.
Graft rejection mediated by antibody recognition of the Gal epitope is a major barrier in porcine to human xenotransplantation. Both NPSCi and PECi cells, cultured on chamber slides, were found to express Gal. The staining intensity on NPSCi cells was similar to that of PECi cells (Fig. 1A, B). Almost all of the NPSCi and PECi cells were Gal positive on analysis with non-fixed single cells by FACS (Fig. 1C, D). This suggests that nearly all of the cells expressed Gal.
The expression of Gal on the surface of NPSCi and PECi cells suggests that they may be recognized by antibodies present in human serum. Therefore, we examined the binding of human IgM on cells using FACS analysis. When cells were incubated with 50% heat-inactivated human type O serum, NPSCi and PECi cells were 76.95±6.03% and 80.76±2.44% positive for human IgM binding, respectively (Fig. 1E, F). No statistical significance was observed (p>0.05). This suggested that the majority of the cells were reactive to human antibodies.
Cells were examined for the formation of MAC by the human antibody and complement system. In contrast to the similar binding of human IgM, NPSCi and PECi cells were 38.41±2.77% and 55.02±5.44% positive for MAC (Fig. 2). MAC formation was significantly lower in NPSCi cells compared to PECi cells (p=0.027). This suggests that NPSCi cells can survive against humoral immune rejection by inhibiting the formation of MAC.
Although the binding of human IgM did not show significant differences between NPSCi and PECi cells, MAC formation was significantly reduced in NPSCi cells. Therefore, we examined whether there are differences in susceptibility to human natural antibody- and complement-mediated lysis between NPSCi and PECi cells using MTT assays (Fig. 3). After 1 hr exposure to human serum in the presence or absence of complement, there were no significant differences in cell lysis between NPSCi and PECi cells, but exposure to normal human serum significantly accelerated the lysis of both cell lines. After 4 hr exposure to heat-inactivated human serum, both cell lines were lysed to some extent but there were no significant differences between cell lines (p>0.05). The percentage of viable NPSCi and PECi cells was not dependent on the length of exposure to heat-inactivated human serum. In contrast, there was a significant decrease in viable PECi cells (12.01±2.50% survival) after incubation with normal human serum. However, NPSCi cells were dramatically resistant to cell lysis compared to PECi cells in the presence of complement (p=0.007). While the viable PECi cells were significantly decreased at 4 hr exposure to normal human serum compared to 1 hr exposure, there was no significant change in the number of viable NPSCi cells.
LPS stimulation strongly increased the expression of surface molecules on DCs such as costimulatory molecules (CD40 and CD80) and MHC II (I-Ab) compared to iDCs (Fig. 4, 5). The increased expression of surface molecules was not found in the abnormally differentiated DCs following the addition of Dexa and D3 (Fig. 4). The addition of Dexa and D3 during initial differentiation from monocytes to DCs strongly decreased the yields of DCs (data not shown) and significantly reduced the expression of I-Ab in the presence of LPS stimulation (data not shown). Co-culture with NPSCi cells did not promote the activation of DCs compared to their activation in the presence of LPS (Fig. 4). This may suggest that NPSCi cells are ignored by xenogeneic DCs and are able to escape the xenogeneic cell-mediated immune response. In contrast, NPSCi cells suppressed the expression of CD40 on DCs even in the presence of LPS. Such suppression of CD40 expression on DCs was not affected by the NPSCi cells:DCs ratio (1:4 or 1:2).
A significant reduction in the expression of CD40 was also observed upon the addition of iSPNT in the presence of LPS compared to the expression in DCs co-cultured with iSPNT (Fig. 5), which showed a correlation with down-regulation of CD40 expression. These findings suggest that the suppressive effect, shown in a co-culturing experiment, is not dependent on the starvation by added competitor cells. Meanwhile, NPSCi cells and iSPNT did not significantly modulate the expression of CD80 and I-Ab on DCs in the presence of LPS stimulation.
In this study, we tested whether NPSCi cells can survive as xenografts against human antibody- and complement-mediated humoral immune responses in vitro. Additionally, the effects of NPSCi cells and iSPNT on the activation and maturation of xenogeneic mouse bone marrow-derived DCs were evaluated.
Although both NPSCi and PECi cells expressed Gal similarly and human IgM reacted with these cells (Fig. 1), NPSCi cells were dramatically resistant to cell lysis compared to PECi cells in the presence of complement as compared to the protective effects of down-regulation of Gal on porcine endothelial cells by RNA interference to protect them from the humoral immune response (17). These results raised the possibility that NPSCi cells can be used as vehicles. The ability of NPSCi cells to survive human antibody- and complement-mediated lysis could be an active process due to the production of immunoprotective factors by SCs. This would suggest that NPSCi cells are able to survive against complement activation by producing one or more immunoprotective factors that may be able to inhibit formation of MAC because MAC formation was significantly inhibited (Fig. 2). Consistent with this observation, SCs are also known to produce clusterin (2, 3), which has been shown to inhibit formation of the MAC in vitro (18). An alternative mechanism is that NPSCi cells survive complement-mediated cytolysis by producing a combination of complement inhibitors. In addition to clusterin, SCs have been shown to produce many immunoprotective factors such as mRNA for complement inhibitors such as the membrane cofactor protein (MCP), protectin (CD59), and decay-accelerating factor (DAF). Previously, it was speculated that complement inhibitors are species-specific, and therefore, porcine complement inhibitors expressed by porcine tissues may be unable to inhibit human complement cytolysis. However, more recent studies indicate that porcine MCP, CD59, and DAF are all effective in inhibiting human complement at least in vitro (5, 6, 19-23).
In general, successful initiation of the cellular immune response requires the activation and maturation of DCs, instruction of naive T cells, and coordination of the primary immune response. However, our results showed that NPSCi cells can suppress the activation and maturation of DCs in the presence of LPS stimulation, suggesting their skew towards an immature or inadequate maturation status. Co-culturing with NPSCi cells or the addition of iSPNT induced impaired maturation of DCs in the presence of LPS stimulation, as shown by suppressed expression of CD40, which is needed for normal antigen presentation to produce an immune response. These novel findings, as we know, provide new insight into the immunopharmacological role of soluble factors from NPSCi cells in impacting the DCs. It is well known that when antigen presentation occurs in the absence of costimulators, a state of anergy can develop, or the preferential induction of antibody-inducing Th2 responses may arise (24). Expression of CD40 on DCs is an especially important factor that determines whether priming will result in immunity or tolerance (25). Antigen-exposed DCs that lack CD40 prevent T cell priming, suppress previously primed immune responses, and induce IL-10 secreting CD4 Tr cells that can transfer antigen-specific tolerance to primed recipients (26). Additionally, a lack of activation of CD40 on DCs by CD40Ldefective anergic cells might be the primary event involved in T cell suppression and supports the role of CD40 signaling in regulating both the activation and survival of DCs (27).
Although the mechanisms responsible for these phenomena are not clear, we can consider the soluble factors secreted by SCs. We previously found that NPSCi cells express TGF β1 and IL-6 (2), which are known to confer tolerogenic properties to DCs and to inhibit development of DCs (28) or lead to the generation of phenotypically mature but functionally impaired DCs (29).
In conclusion, our results raised the possibility that NPSCi cells can be engineered using transgenic technology to produce useful proteins or can be used as a vehicle. NPSCi cells can also convert APCs into an inhibitory or suppressor phenotype via secreting immune modulatory proteins, thus locking them into a semi-mature state, thereby resulting in an attenuated or regulatory T cell response. This opens optimistic perspectives for the use of porcine SCs for modulation of cell-mediated immune responses compared to previous DC-based tolerance induction technologies that have limitations including the debilitating consequences of the use of lifelong immunosuppressant regimens. More studies are needed to address the in vivo effect of porcine SCs on the induction of peripheral tolerance.
Figures and Tables
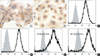 | Fig. 1Expression of Gal-α1,3-Gal (Gal) on immortalized porcine Sertoli (NPSCi) or aortic endothelial (PECi) cells. Cells cultured as a monolayer on chamber slides and fixed for 2 hr with absolute cold ethanol were immunostained for IB4 lectin (brown). Most of NPSCi (A) and PECi (B) cells are positive to the Gal epitope. FACS analysis of non-fixed NPSCi (C) and PECi (D) cells stained with FITC-conjugated IB4 lectin (1:50) for 1 hr to detect Gal (solid line). Also, there are no significant differences in the binding of human IgM to binding of human xenoreactive antibodies to NPSCi (E, 76.95±6.03%) or PECi (F, 80.76±2.44%) cells (p>0.05). |
 | Fig. 2Formation of membrane attack complex (MAC) on the immortalized porcine Sertoli (NPSCi, A, 38.41±2.77%) and aortic endothelial (PECi, B, 55.02±5.44%) cells in the presence of normal human serum. Both cell lines show the significant difference in the formation of MAC (p=0.027). Gray histograms correspond to the control cells that were not pre-incubated with human serum, and solid histograms represent specific staining of human C5b-9. The data are presented as mean±SD of three individual experiments in triplicate. |
 | Fig. 3Susceptibility of immortalized porcine Sertoli (NPSCi) or aortic endothelial (PECi) cells to human natural antibody- and complement-mediated lysis. Negative control values (media alone) for NPSCi and PECi cells were arbitrarily set equal to 100%, and the relative percentage of cell viability was calculated for each condition. The data are presented as the mean±SD of three individual experiments in triplicate. Means bearing different superscript letters are significantly different at p<0.05.
M, media alone; M+C, media+rabbit complement; iNHS, heat-inactivated normal human serum; NSH, normal human serum.
|
 | Fig. 4The effects of immortalized porcine Sertoli cells on the activation of dendritic cells. Immortalized porcine SCs (NPSCi cells) do not activate DCs (empty bar) but inhibit cell surface expression of CD40 in the presence of LPS. The data are presented as the mean±SD of three individual experiments in triplicate. Means bearing different superscript letters are significantly different at p<0.05.
MFI, mean fluorescence index; ciDC, immature DC differentiated in the presence of Dexa and D3; iDC, immature DC; LPS, lipopolysaccharide.
|
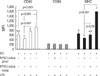 | Fig. 5The effects of culture supernatant of immortalized porcine Sertoli cells (iSPNT) on the activation of dendritic cells (DCs) in the presence of LPS. CD40 expression on DCs was significantly inhibited in the presence of iSPNT. But iSPNT did not affect the expression of CD80 and I-Ab molecules on the surface of LPS stimulated DCs. The data are presented as the mean±SD of three individual experiments in triplicate. Means bearing different superscript letters are significantly different at p<0.05.
MFI, mean fluorescence index; iDC, immature DC; LPS, lipopolysaccharide.
|
ACKNOWLEDGEMENT
The authors thank, Jae Min Lee is for his help with the overall miscellaneous works as a part-time educatee.
References
1. Dufour JM, Rajotte RV, Seeberger K, Kin T, Korbutt GS. Long-term survival of neonatal porcine Sertoli cells in non-immunosuppressed rats. Xenotransplantation. 2003. 10:577–586.

2. Lee HM, Oh BC, Lim DP, Lee DS, Cho J, Lee G, Lee JR. Establishment and characterization of porcine Sertoli cell line for the study of xenotransplantation. Xenotransplantation. 2007. 14:112–118.

3. Lee HM, Oh BC, Lim DP, Lee DS, Cho J, Lee G, Lee JR. Role of complement regulatory proteins in the survival of murine allo-transplanted Sertoli cells. J Korean Med Sci. 2007. 22:277–282.

4. Bailey R, Griswold MD. Clusterin in the male reproductive system: localization and possible function. Mol Cell Endocrinol. 1999. 151:17–23.

5. van den Berg CW, Morgan BP. Complement-inhibiting activities of human CD59 and analogues from rat, sheep, and pig are not homologously restricted. J Immunol. 1994. 152:4095–4101.
6. van den Berg CW, Perez de la Lastra JM, Llanes D, Morgan BP. Purification and characterization of the pig analogue of human membrane cofactor protein (CD46/MCP). J Immunol. 1997. 158:1703–1709.
7. Suarez-Pinzon W, Korbutt GS, Power R, Hooton J, Rajotte RV, Rabinovitch A. Testicular sertoli cells protect islet beta-cells from autoimmune destruction in NOD mice by a transforming growth factor-beta1-dependent mechanism. Diabetes. 2000. 49:1810–1818.

8. Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995. 377:630–632.

9. Yang H, Wright JR Jr. Co-encapsulation of Sertoli enriched testicular cell fractions further prolongs fish-to-mouse islet xenograft survival. Transplantation. 1999. 67:815–820.
10. Halberstadt C, Emerich DF, Gores P. Use of Sertoli cell transplants to provide local immunoprotection for tissue grafts. Expert Opin Biol Ther. 2004. 4:813–825.

11. Reis e Sousa C. Activation of dendritic cells: translating innate into adaptive immunity. Curr Opin Immunol. 2004. 16:21–25.

12. Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001. 106:255–258.
13. Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003. 21:685–711.
14. Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003. 196:125–146.

15. Dufour JM, Hamilton M, Rajotte RV, Korbutt GS. Neonatal porcine Sertoli cells inhibit human natural antibody-mediated lysis. Biol Reprod. 2005. 72:1224–1231.
16. Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, Zugel U, Steinmeyer A, Pollak A, Roth E, Boltz-Nitulescu G, Spittler A. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006. 36:361–370.
17. Zhu M, Wang SS, Xia ZX, Cao RH, Chen D, Huang YB, Liu B, Chen ZK, Chen S. Inhibition of xenogeneic response in porcine endothelium using RNA interference. Transplantation. 2005. 79:289–296.

18. Jenne DE, Tschopp J. Molecular structure and functional characterization of a human complement cytolysis inhibitor found in blood and seminal plasma: identity to sulfated glycoprotein 2, a constituent of rat testis fluid. Proc Natl Acad Sci USA. 1989. 86:7123–7127.

19. Kirszbaum L, Sharpe JA, Murphy B, d'Apice AJ, Classon B, Hudson P, Walker ID. Molecular cloning and characterization of the novel, human complement-associated protein, SP-4040: a link between the complement and reproductive systems. EMBO J. 1989. 8:711–718.

20. Bozas SE, Kirszbaum L, Sparrow RL, Walker ID. Several vascular complement inhibitors are present on human sperm. Biol Reprod. 1993. 48:503–511.
21. Hinchliffe SJ, Rushmere NK, Hanna SM, Morgan BP. Molecular cloning and functional characterization of the pig analogue of CD59: relevance to xenotransplantation. J Immunol. 1998. 160:3924–3932.
22. Maher SE, Pflugh DL, Larsen NJ, Rothschild MF, Bothwell AL. Structure/function characterization of porcine CD59: expression, chromosomal mapping, complement-inhibition, and costimulatory activity. Transplantation. 1998. 66:1094–1100.
23. Perez de la Lastra JM, Harris CL, Hinchliffe SJ, Holt DS, Rushmere NK, Morgan BP. Pigs express multiple forms of decay-accelerating factor (CD55), all of which contain only three short consensus repeats. J Immunol. 2000. 165:2563–2573.

24. Thompson CB. Distinct roles for the costimulatory ligands B7-1 and B7-2 in T helper cell differentiation? Cell. 1995. 81:979–982.

25. Hanninen A, Martinez NR, Davey GM, Heath WR, Harrison LC. Transient blockade of CD40 ligand dissociates pathogenic from protective mucosal immunity. J Clin Invest. 2002. 109:261–267.
26. Martin E, O'Sullivan B, Low P, Thomas R. Antigen-specific suppression of a primed immune response by dendritic cells mediated by regulatory T cells secreting interleukin-10. Immunity. 2003. 18:155–167.

27. Frasca L, Scotta C, Lombardi G, Piccolella E. Both maturation and survival of human dendritic cells are impaired in the presence of anergic/suppressor T cells. Clin Dev Immunol. 2003. 10:61–65.

28. Brown RD, Pope B, Murray A, Esdale W, Sze DM, Gibson J, Ho PJ, Hart D, Joshua D. Dendritic cells from patients with myeloma are numerically normal but functionally defective as they fail to up-regulate CD80 (B7-1) expression after huCD40LT stimulation because of inhibition by transforming growth factor-beta1 and interleukin-10. Blood. 2001. 98:2992–2998.
29. Hegde S, Pahne J, Smola-Hess S. Novel immunosuppressive properties of interleukin-6 in dendritic cells: inhibition of NF-kappaB binding activity and CCR7 expression. FASEB J. 2004. 18:1439–1441.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download