Abstract
Figures and Tables
 | Fig. 1Slit lamp findings of donor cornea and structural findings of in vivo expanded corneal epithelium on donor cornea at 2 weeks of cultivation (case 1). (A) Denuded AM was transplanted onto the cornea using a continuous suture with #10-0 Nylon after epithelial peeling. Epithelialization on the AM was complete by 2 weeks after the AM transplantation. (B) HE staining demonstrated well differentiated multilayer epithelium in the in vivo expanded epithelial sheet under the light microscopy (×200). (C) EM showed a well developed hemidesmosome (arrows). |
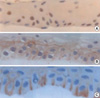 | Fig. 2Immunohistochemical staining of p63 (A), Connexin 43 (B) and Integrin β4 (C) from the corneal epithelial culture of the donor's cornea at 2 weeks of cultivation (case 1) (×400).
(A) p63 was expressed by the epithelial basal cells. (B) Connexin 43 was expressed in the intercellular space. (C) Integrin β4 was expressed in the intercellular space, predominantly the basal cell layer.
|
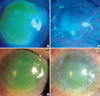 | Fig. 3A 44-yr-old patient with Steven Johnson syndrome (case 1) had PED (8 mm diameter), present for more than 5 months (A, C). However, the epithelial defect was completely covered with the in vivo expanded epithelial sheet 2 weeks after surgery (B, D). |
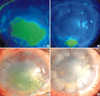 | Fig. 4A 37-yr-old patient with alkali burns (case 3) had PED, presented for more than 5 months (A, C). The epithelial defect was completely covered, but erosion in another area developed 2 weeks after surgery (B, D). |
Table 1

Clinical success was defined by the epithelium of cornea recovery and maintenance of an intact epithelial surface for 4 weeks. According to this definition of clinical success, the clinical success rate of these cases were 100% and this procedure seems to be very effective. In cases 1 and 2, the ocular surface recovered for 4 weeks. However, the epithelial defect recurred because of chronic inflammation and chronic exposure, respectively. In case 3, the cornea recovered after surgery, was maintained for 27 weeks and followed by PKP.
SJS, Steven-Johnson syndrome; PED, persistent epithelial defect; CLAU, conjunctivolimbal autograft; KLAL, keratolimbal allograft; PKP, penetrating keratoplasty.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download