Abstract
Mild cognitive impairment (MCI) has been defined as a transitional state between normal aging and Alzheimer disease. Diffusion tensor imaging (DTI) can estimate the microstructural integrity of white matter tracts in MCI. We evaluated the microstructural changes in the white matter of MCI patients with DTI. We recruited 11 patients with MCI who met the working criteria of MCI and 11 elderly normal controls. The mean diffusivity (MD) and fractional anisotropy (FA) were measured in 26 regions of the brain with the regions of interest (ROIs) method. In the MCI patients, FA values were significantly decreased in the hippocampus, the posterior limb of the internal capsule, the splenium of corpus callosum, and in the superior and inferior longitudinal fasciculus compared to the control group. MD values were significantly increased in the hippocampus, the anterior and posterior limbs of the internal capsules, the splenium of the corpus callosum, the right frontal lobe, and in the superior and the inferior longitudinal fasciculus. Microstructural changes of several corticocortical tracts associated with cognition were identified in patients with MCI. FA and MD values of DTI may be used as novel biomarkers for the evaluation of neurodegenerative disorders.
Mild cognitive impairment (MCI) has been defined as a transitional state between normal aging and Alzheimer disease (AD). Individuals with MCI do not meet diagnostic criteria for dementia, but MCI is associated with a significantly increased risk of developing AD compared to the elderly population without cognitive impairment (1-4). Many studies found the conversion rates ranging from 3% to 40% per year (5). There have been several studies on the structural and functional changes that precede the clinical diagnosis of AD, and on the development of sensitive neuroimaging biomarkers to predict conversion to AD. Volume changes of the mesial temporal structures such as the hippocampus and the entorhinal cortex, and metabolic changes of the posterior cingulate have been identified as important biomarkers (6-8). Although most of the previous imaging studies, on MCI and mild AD, have focused on the structural or metabolic changes of graymatter, white matter (WM) alterations may also be an important component of onset and progression of disease.
Cognitive functions such as attention and memory depend on the cooperation of multiple brain regions that are connected by fibers of WM. Various neuronal degenerative diseases, and age-related cognitive impairments, are assumed to be related to the disconnection of these neuronal networks. Therefore, the evaluation of the WM tracts that connect functionally essential cortical areas for cognition is important for the study of dementia. The new magnetic resonance (MR) technique, diffusion tensor imaging (DTI), might add to our knowledge of ultrastructure changes of white matter in the brain. The direction of the movement of water molecules into tissues is determined by many factors including cell membranes, axonal membranes, and cytoskeletal structures such as neurofilaments and microtubules. The relative preference of the direction of diffusion is expressed as the fractional anisotropy (FA), which is used as a measure of WM integrity. A reduction of FA values suggests a reduction in axon number, an impairment of axonal flow, or both. The mean diffusivity (MD) is a measure of random mean water diffusion. It can be used as a measure of alteration of brain tissue. Increased MD most likely results from the loss of neurons, axons, or dendrites, which results in an increase in extracellular space and increased water diffusion. In AD, a decline in FA and an increase in MD for the corpus callosum, frontal, parietal, and occipital lobes has been demonstrated due to the structural changes of the WM (9-12).
MCI has been considered as a preclinical AD state. We investigated whether the WM damage identified in AD could be demonstrated in MCI by using DTI. We also evaluated which regions of WM damage appeared to be important for the development of MCI.
Eleven patients (6 women and 5 men; mean age 72.64 yr; range, 60-88 yr) who met the working criteria for MCI based on Peterson et al. and the others were recruited as detailed in Table 1 (13-15). We characterized memory complaint by at least a 3-month history of memory problems. Perterson et al. did not specify which tests should be used to measure verbal or performance IQ. We explicitly stated that Korean version of Mini-Mental State Exam (K-MMSE) was used to operationalize the third criterion of normal cognition (26). In our study, a cut-off was rigorously applied for 1.5 standard deviation (SD) above age and educational appropriate norm. The Clinical Dementia Rating (CDR) scale, broadly accepted by clinicians as a good staging tool for dementia severity, consists of six subscales: memory, orientation, judgement and problem solving, community affairs, home and hobbies, and personal care (27). We defined MCI as a rating of 0.5 CDR. For the fourth criterion of abnormal memory, the episodic memory score of Seoul Verbal Learning Test (SVLT) was used because it is widely used in clinical neuropsychological assessment and has available norms (28). A cut-off was applied 1.5 SD below age and educational appropriate norm. Peterson et al. did not specify measures to assess activities of daily living (ADL) nor acceptable performance levels (14, 15). So we used the Korean Instrumental Activities of Daily Living (K-IADL) to measure functional disabilities. The K-IADL consists of 11 items that were selected by the Representative Committee of the Korean Dementia Research Group. These items include shopping, mode of transportation, ability to handle finances, house keeping, preparing food, ability to use a telephone, responsibility for taking medication, recent memory, hobbies, watching television, and fix up around the house. The informants rated each item as follows: 0=normal, 1=with some assistance, 2=with much assistance, 3=unable to do, and NA=not appilicable. The total is devided by numbers of the items except NA. With a cut-off point of 0.43, the K-IADL had a sensitivity of 83% and a specificity of 82% in the diagnosis of dementia (29, 30). For the criterion of normal activity of daily living cut-off scoes less than 0.43 was used.
Exclusion criteria for the MCI patients were evidence of other neurological, psychiatric, or systemic conditions that could cause cognitive impairment (e.g., stroke, tumor, alcoholism, major depression, and a history of temporal epilepsy). In addition, we excluded MCI patients who showed deep WM hyperintensity signals rated as Fazekas grade 2 (beginning confluence of foci) or above on the MR imaging findings (28). Four of the eleven MCI patients had some punctuate foci defined as Fazenkas grade 1 and all others were grade 0.
The age-, gender-, and education-matched healthy controls (5 women and 6 men; mean age, 70.64 yr, range, 60-76 yr) were recruited from volunteers. The controls had no complaint of memory loss and no evidence of cognitive deficits on interview and the K-MMSE.
We performed DTI using a 1.5-Tesla magnetic resonance imager (Signa Excite 11.0, General Electric Medical Systems) with a single-shot, spin echo, echo planar, diffusion-weighted sequence in addition to the full conventional MRI. Headmotion was minimized by the use of tightly padded clamps attached to the head coil. A high-resolution brain coil was used for the transmission of radio frequency pulses and signal reception. A series of axial diffusion-weighted images with a diffusion sensitizing gradient (b-value=1,000 sec/mm2) along 25 directions was obtained, as well as the acquisition of axial images without diffusion weighting (b-value=0). The other diffusion parameters were as follows: time of repetition (TR)=10,000 ms, time of echo (TE)=78 ms, matrix=128×128 mm, field of view (FOV)=260×260 mm, number of excitations (NEX)=1, 35 axial slices and a slice thickness=4 mm with no interslice gap. The signal to noise ratio, (NSR0), in the reference (b-0) image was about 100 (%), which helped to reduce the bias in the estimated magnetization transfer imaging (MTI) metrics.
The raw DTI data were transferred to a personal computer equipped with the freely shared programs Volume-One (version 1.56) and dTV (version II). These programs are available online at www.volume-one.org and www.utradiology.umin.jp/people/masutani/dTV.htm, respectively. In addition to the b0 and b1000 images (isotropic DWI), diffusion tensor maps of the FA, MD, and color-coded images were generated. The diffusion tensor parameters were calculated on a voxel-by-voxel basis (1×1×3.8 mm), and the diffusion eigenvectors and corresponding eigen values (×1, ×2, ×3) were then acquired. An eigen vector (e1) associated with the largest eigen value (×1) was assumed to represent the local fiber direction.
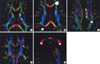
A standardized placement procedure was used to place the spherical regions of interest (ROIs), using atlas-based rules with morphological landmarks by Mori et al. for each MRI (25). The ROIs for the cerebral WM were selected in areas of maximal distance from the cortical gray matter and from the ventricle, to avoid partial volume averaging. MD and FA values were measured in the ROIs of variable sizes in several WM regions. ROIs were placed bilaterally in the normal appearing WM of the following areas including the hippocampus, a part of GM: the genu, the body, and the splenium of the corpus callosum, the temporal lobe, the superior and inferior longitudinal fasciculus, the anterior and posterior cingulates, the parietal, frontal, and occipital WM, the anterior and posterior limbs of internal capsule, the corona radiata, the centrum semiovale, and so on. The temporal lobe ROIs were placed on the temporal stem. The hippocampus ROIs were placed in the center of the hippocampal heads, and its location was confirmed on the axial and coronal slices in order to avoid perihippocampal cerebrospinal fluid contamination. In order to place the anterior and posterior cingulate ROIs, we displayed the color maps. In this way, the cingulum could be identified and differentiated from the callosal radiation. Each of the ROIs was placed in the brightest green areas of the anterior and posterior parts of the cingulate fiber tracts (Fig. 1C). The parietal lobe ROIs could be found on -45 degree and 35 mm from the ipsilateral posterior cingulate. The anterior and posterior limbs of internal capsule ROIs were placed in the midlines of each limb. The corpus callosum ROIs were placed in the centers of its parts (the genu, the body, and the splenium). The superior and inferior longitudinal fasciculus were identified after fiber tracking on this color coded DTI image (Fig. 1D). The voxel size of the ROI was determined as 7, 25, 181, and 318 µL, according to the size of the region. The 7 µL voxel was applied to the hippocampus, the temporal lobe, the anterior and posterior limbs of the internal capsule, the body of the corpus callosum, superior and inferior longitudinal fasciculus as well as the anterior and posterior cingulates. The 25 µL voxel was applied to the genu and splenium of the corpus callosum. The 181 µL voxel was applied to the parietal and occipital WMs. The 318 µL voxel was applied to the frontal WM. A total of 26 ROIs were placed on the DTI images.
Two experienced investigators, blinded to the diagnosis, measured hippocampal FA and MD values of both sides four times of all patients and controls in random order. These areas were chosen because they are particularily difficult to evaluate due to the susceptibility artifacts. Inter- and intrarater reliability revealed stable results with a highly satisfactory correlation (Spearman's rho): the mean interrater correlation was 0.88, and the intrarater correlations were 0.91 and 0.96 for two investigators, respectively.
The demographic data from the two groups were analyzed with the Wilcoxon rank sum test and Fisher's exact test for categorical variables. The FA and MD values of all 58 areas were compared respectively by the Wilcoxon rank sum test with a p value of <0.05. Statistical analyses were performed with the SPSS software package version 13.0 (SPSS, Chicago, IL, U.S.A.).
Demographic and cognitive status information on the participants are listed in Table 2. Mean age, education, and gender were not significantly different between MCI patients and the controls. However, the K-MMSE score was significantly lower in the MCI group (p<0.005).
For the MCI patients, FA values were significantly decreased in the hippocampus, the posterior limb of internal capsule, the splenium of the corpus callosum, the superior and inferior longitudinal fasciculus compared to the controls as detailed in Table 3. MD values were significantly increased in the hippocampus, the anterior and posterior limbs of internal capsule, the splenium of the corpus callosum, the right frontal lobe, the superior and inferior longitudinal fasciculus as detailed in Table 4. FA and MD indices for the other areas (including the temporal, the occipital, the anterior and posterior cingulates, genu and body of the corpus callosum, the centrum semiovale, and so on) were not significantly different in comparisons between the MCI patients and controls.
As AD progresses, neuronal and pathologic changes typically start in the hippocampus and the entorhinal areas and then expand into the temporal and parietal association cortices (8-11, 17). MCI has been defined as the preclinical state of AD, and even though MCI patients have no definite difficulties in their social and occupational activities, they have similar pathologic changes as do AD patients (5-7). In this study, all MCI patients had normal-appearing WM on conventional MRI (Fazekas grade 0 or 1). However, we found microstructural changes of WM in patients with MCI by using DTI analysis.
During neurodegeneration, neuronal loss leads to an incremental increase in water molecules that can be quantified by increased MD values. In addition, the pathologic processes that lead to disturbances of WM homogeneity and directionality can be expressed by decreased FA values. The damage to the WM in MCI patients was likely associated with pathological processes of direct or indirect effects of neurofibrillary tangles (NFTs) and amyloid plaques (SPs) in the cortex and WM fibers. NFTs are mainly found in cortical layers III and V and impair the large glutaminergic projection neurons (15, 16). Damage of the glutaminergic projection neurons, which connect cortical or subcortical areas, may cause degeneration of major WM tracts. SPs accumulated predominantly in layers II and III in the association areas of the neocortex and affect large pyramidal cells that provide input/output functions (16).
Our DTI results are consistent with those of previous studies demonstrating abnormal WM integrity in MCI and AD (3, 5-7). Previous studies have shown that WM changes in the corpus callosum, temporal lobe, parietal lobe, the cingulum, and the superior longitudinal fasciculus (8, 11, 17). The location of abnormalities of WM may differ across studies depending upon the exact placement of the ROIs. Of note from the findings of our study were the significant FA and MD changes in the splenium of the corpus callosum and the superior and inferior longitudinal fasciculus.
The corpus callosum is one of the most heavily myelinated regions of the brain. It consists of fibers arising from the large pyramidal neurons in layers III and V, which transmit interhemispheric corticocortical fibers. Several MRI studies have investigated global and regional size reduction of the corpus callosum in patients affected with various neurodegenerative disorders including AD, vascular dementia, progressive supranuclear palsy, and amyotrophic lateral sclerosis (21, 22). Atrophy of the corpus callosum in a variety of brain disorders may occur as a result of Wallerian degeneration due to the cortical lesions with loss of interhemispheric fibers. The splenium of the corpus callosum consists of fiber tracts that connect the temporo-parieto-occipital cortex. Therefore, the interhemispheric corticocortical disconnection related to the splenial change contributes to cognitive impairment. Other studies using DTI, diffusion-weighted and magnetization transfer (MT) imaging have found a significant reduction in the structural integrity of WM in the splenium of AD patients (23, 24). Involvement of the splenium in our study complements the results of the previous studies and suggests early involvement of splenium in MCI patients prior to the development of overt AD.
The superior longitudinal fasciculus connects the frontal and parietal lobes, and the inferior longitudinal fasciculus connects the frontal, parietal, and medial temporal lobes. They convey sensory, visual, auditory, and somatosensory information from the posterior part of the brain to the anterior part (24). Therefore, these WM projections may play an important role in the memory, attention, and executive functions. Thus, disconnection of the superior and inferior longitudinal fasciculus may cause impaired information flow to the frontal and temporal lobe and visa versa, and may explain memory, attention, and executive dysfunction in MCI patients.
Our study had several limitations. The ROI approach is not optimal to map the whole brain WM. Partial volume effects of GM and/or CSF in the WM samples may blur DTI measures, most notably the inferior frontal and inferior temporal regions. Therefore, the ROI approach may be subjective with inconsistent definitions of anatomical borders across studies and poor reproducibility. A voxel-based statistical approach or statistical parametric mapping (SPM) analysis will be needed in future studies. DTI is a unique tool to visualize and segment the WM pathways but not specific analysis because fibers are connected with each other complicatedly. So we used atlas-based rules with morphological landmarks by Mori et al. and color-coded DTI image for detection of the specific tracts. Additionally, the study was limited by the small number of subjects involved. Future work on larger samples of MCI, early AD patients, and controls may provide more information on the significance of WM changes in the pathogenesis of neurodegeneration. In addition, longitudinal research on MCI patients will help determine the specific sites of WM changes in those who progress to overt AD and those who do not; which may provide a new biomarker for dementia. Finally, further evaluation including the relationship of the neuropsychological scores and neuropathology has to be investigated.
In conclusion, DTI, an indicator of fiber tract integrity, provides a more direct assessment of the neuroanatomical configuration of WM fiber tracts. We found significant microstructural changes of WM tracts in several corticocortical and corticosubcortical connection areas that are associated with cognition in patients with MCI before the onset of overt dementia by using DTI analysis. The abnormal FA and MD values for these tracts may be used as new biomarkers for the evaluation of MCI or AD.
Figures and Tables
Fig. 1
Illustrations of ROI positioned on color-coded images. Fiber orientations are assigned specific colors, as follows: red, the right-to-left orientation; green, the anterior-to-posterior orientation; and blue, the superior-to-inferior orientation.
(A) Anterior and posterior limb of internal capsule, (B) frontal and occipital lobe, (C) anterior and posterior cingulate, parietal lobe, (D) superior and inferior logitudinal fasciculus, temporal lobe, (E) genu, body, and splenium of corpus callosum.
References
1. Medina D, DeToledo-Morrell L, Urresta F, Gabrieli JD, Moseley M, Fleischman D, Bennett DA, Leurgans S, Turner DA, Stebbins GT. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging. 2006. 27:663–672.

2. Fellgiebel A, Muller MJ, Wille P, Dellani PR, Scheurich A, Schmidt LG, Stoeter P. Color-coded diffusion-tensor-imaging of posterior cingulate fiber tracts in mild cognitive impairment. Neurobiol Aging. 2005. 26:1193–1198.

3. Fellgiebel A, Wille P, Muller MJ, Winterer G, Scheurich A, Vucurevic G, Schmidt LG, Stoeter P. Ultrastructural hippocampal and white matter alterations in mild cognitive impairment: a diffusion tensor imaging study. Dement Geriatr Cogn Disord. 2004. 18:101–108.

4. Charlton RA, Barrick TR, McIntyre DJ, Shen Y, O'Sullivan M, Howe FA, Clark CA, Morris RG, Markus HS. White matter damage on diffusion tensor imaging correlates with age-related cognitive decline. Neurology. 2006. 66:217–222.

5. Geslani DM, Tierney MC, Herrmann N, Szalai JP. Mild cognitive impairment: an operational definition and its conversion rate to Alzheimer's disease. Dement Geriatr Cogn Disord. 2005. 19:383–389.

6. Muller MJ, Greverus D, Dellani PR, Weibrich C, Wille PR, Scheurich A, Stoeter P, Fellgiebel A. Functional implications of hippocampal volume and diffusivity in mild cognitive impairment. Neuroimage. 2005. 28:1033–1042.

7. Hirata Y, Matsuda H, Nemoto K, Ohnishi T, Hirao K, Yamashita F, Asada T, Iwabuchi S, Samejima H. Voxel-based morphometry to discriminate early Alzheimer's disease from controls. Neurulet. 2005. 382:269–274.

8. Masdeu JC, Zubieta JL, Arbizu J. Neuroimaging as a marker of the onset and progression of Alzheimer's disease. J Neurol Sci. 2005. 236:55–64.

9. Vrenken H, Pouwels PJ, Geurts JJ, Knol DL, Polman CH, Barkhof F, Castelijin JA. Altered diffusion tensor in multiple sclerosis normal-appearing brain tissue: cortical diffusion changes seem related to clinical deterioration. J Magn Reson Imaging. 2006. 23:628–636.

10. Lee FK, Fang MR, Antonio GE, Yeung DK, Chan ET, Zhang LH, Yew DT, Ahuja AT. Diffusion tensor imaging (DTI) of rodent brains in vivo using a 1.5T clinical MR scanner. J Magn Reson Imaging. 2006. 23:747–751.

11. Mukherjee P, McKinstry RC. Diffusion tensor imaging and tractography of human brain development. Neuroimaging Clin N Am. 2006. 16:19–43.

12. Gulani V, Sundgren PC. Diffusion tensor magnetic resonance imaging. J Neuroophthalmol. 2006. 26:51–60.

13. Modrego PJ. Predictors of conversion to dementia of probable Alzheimer type in patients with mild cognitive impairment. Curr Alzheimer Res. 2006. 3:161–170.

14. Hodges JR, Erzinclioglu S, Patterson K. Evolution of cognitive deficits and conversion to dementia in patients with Mild Cognitive Impairment: a very-long-term follow-up study. Dement Geriatr Cogn Disord. 2006. 21:380–391.

15. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging memory, and mild cognitive impairment. Int Psychogeriatr. 1997. 9:Suppl 1. 65–69.

16. Sun SW, Song SK, Harms MP, Lin SJ, Holtzman DM, Merchant KM, Kotyk JJ. Detection of age-dependent brain injury in a mouse model of brain amyloidosis associated with Alzheimer's disease using magnetic resonance diffusion tensor imaging. Exp Neurol. 2005. 191:77–85.

17. Takahashi S, Yonezawa H, Takahashi J, Kudo M, Inoue T, Tohgi H. Selective reduction of diffusion anisotropy in white matter of Alzheimer disease brains measured by 3.0 Tesla magnetic resonance imaging. Neurulet. 2002. 332:45–48.

18. Rose SE, Chen F, Chalk JB, Zelaya FO, Strugnell WE, Benson M, Semple J, Doddrell DM. Loss of connectivity in Alzheimer's disease: an evaluation of white matter tract integrity with color coded MR diffusion tensor imaging. J Neurol Neurosurg Psychiatry. 2000. 69:528–530.
19. Bozzali A, Falini M, Franceschi M, Cercignani M, Zuffi G, Scotti G, Commi G, Filippi M. White matter damage in Alzheimer's disease resonance imaging assessed in vivo using diffusion tensor magnetic. J Neurol Neurosurg Psychiatry. 2002. 72:742–746.
20. Bozzali M, Franceschi M, Falini A, Pontesilli S, Cercignani M, Magnani G, Scotti G, Comi G, Filippi M. Quantification of tissue damage in AD using diffusion tensor and magnetization transfer MRI. Neurology. 2001. 57:1135–1137.

22. Pantel J, Schröder J, Jauss M, Essig M, Minakaran R, Schönknecht P, Schneider G, Schad LR, Knopp MV. Topography of callosal atrophy reflects distribution of regional volume reduction in Alzheimer's disease. Psychiatry Res. 1999. 90:181–192.
23. Hampel H, Teipel SJ, Alexander GE, Horwitz B, Teichberg D, Schapiro MB, Rapoport SI. Corpus callosum atrophy is a possible indicator of region-and cell type-specific neuronal degeneration in Alzheimer disease: a magnetic resonance imaging analysis. Arch Neurol. 1998. 55:193–198.
24. Wang PJ, Saykin AJ, Flashman LA, Wishart HA, Rabin LA, Santulli RB, McHugh TL, MacDonald JW, Mamourian AC. Regionally specific atrophy of the corpus callosum in AD, MCI and cognitive complaints. Neurobiol Aging. 2006. 27:1613–1617.

25. Mori S, Wakana S, Nagae-Poetscher LM, Van Zijl P. MRI Atlas of human white matter. 2005. Amsterdam, Netherlands: Elsevier;11–30.
26. Kang Y, Na DL, Han S. A validity study on the Korean mini-mental state examination (K-MMSE) in dementia patients. J Korean Neurol Assoc. 1997. 15:300–308.
27. Choi SH, Na DL, Lee BH, Hahm DS, Jeong JH, Yoon SJ, Yoo KH, Ha CK, Han IW. Estimating the validity of Korean version of extended clinical dementa rating (CDR) Scale. J Korean Neurol Assoc. 2001. 19:585–591.
28. Kwak YT, Cho DS. Usefulness of Seoul verbal learning test in differential diagnosis of Alzheimer's disease and subcortical vascular dementia. J Korean Neurol Assoc. 2004. 22:22–28.
29. Kang SJ, Choi SH, Lee BH, Kwon JC, Na DL, Han SH; Korean Dementia Research Group. The reliability and validity of the Korean Instrumental Activities of Daily Living (K-IADL). J Korean Neurol Assoc. 2002. 20:8–14.
30. Kim DS, Hahm DS, Kwak YT, Han IW. Neuropsychological differentiation between Alzheimer's disease and vascular dementia. J Korean Neurol Assoc. 2001. 19:143–148.
31. Sohn EH, Lee AY, Yu SD, Kwon DH, Kim TW, Kim JM. Analysis of causative factors and effects to cognitive functions of cerebral white matter changes. J Korean Neurol Assoc. 2001. 19:471–477.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download