Abstract
We developed nomograms to predict disease recurrence in patients with Ta, T1 transitional cell carcinoma of the bladder. Thirty-eight training hospitals participated in this retrospective multicenter study. Between 1998 and 2002, a total of 1,587 patients with newly diagnosed non-muscle invasive bladder cancer were enrolled in this study. Patients with prior histories of bladder cancer, non-transitional cell carcinoma, or a follow-up duration of less than 12 months were excluded. With univariate and multivariate logistic regression analyses, we constructed nomograms to predict disease recurrence, and internal validation was performed using statistical techniques. Three-year and five-year recurrence-free rates were 64.3% and 55.3%, respectively. Multivariate analysis revealed that age (hazard ratio [HR]=1.437, p<0.001), tumor size (HR=1.328, p=0.001), multiplicity (HR=1.505, p<0.001), tumor grade (HR=1.347, p=0.007), concomitant carcinoma in situ (HR=1.611, p=0.007), and intravesical therapy (HR=0.681, p<0.001) were independent predictors for disease recurrence. Based on these prognostic factors, nomograms for the prediction of disease recurrence were developed. These nomograms can be used to predict the probability of disease recurrence in patients with newly diagnosed Ta, T1 transitional cell carcinoma of the bladder. They may be useful for patient counseling, clinical trial design, and patient follow-up planning.
Transitional cell carcinoma (TCC) of the urinary bladder can present as a non-muscle invasive or as a muscle-invasive lesion. The majority of patients (approximately 75%) present with non-muscle invasive tumors, which are limited to the mucosa (Ta) or the lamina propria (T1) (1). Carcinoma in situ (Cis) can also be considered non-muscle invasive. However, Cis tends to behave more aggressively and is often found in association with high-grade non-muscle invasive tumors. Prognostic factors in patients with non-muscle invasive bladder cancer have been the subject of numerous publications for many years (2-18). Depending on a patient's characteristics, the probability of recurrence after transurethral resection (TUR) at one year ranges from about 15% to 70% (2), and the probability of progression at five years ranges from about 7% to 40% (6).
Non-muscle invasive tumors may be managed with TUR with or without intravesical therapy. However, not all non-muscle invasive bladder cancer patients have the same risk for disease recurrence and progression, and the absence of risk stratification between aggressive and indolent tumors contributes to potentially excessive frequent surveillance or unnecessary intravesical therapy. Several studies have suggested risk tables for the recurrence and progression of non-muscle invasive bladder cancer (5, 7, 9, 10), but they cannot calculate the probability of a certain event, such as the probability of recurrence within five years.
The purpose of this study was to develop and validate prognostic nomograms for disease recurrence in patients with Ta, T1 transitional cell carcinomas of the bladder. These nonograms will facilitate patient counseling, choosing the most appropriate adjuvant treatment after TUR, and determining the frequency of follow-up in individual patients.
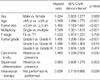
Thirty-eight training hospitals participated in this retrospective multicenter study. Between January 1998 and December 2002, 3,060 patients with newly diagnosed, non-muscle invasive bladder cancer were evaluated. They were treated with transurethral resection (TUR) and diagnosed with Ta or T1 TCC of the bladder based on the 2002 American Joint Committee on Cancer (AJCC) TNM staging system (19). Patients with prior histories of bladder cancer, non-TCC histology, primary Cis, or a follow-up duration of less than 12 months were excluded. This cohort study was also limited to tumors whose grades were determined using the 1973 World Health Organization system. After exclusion, 1,587 patients were enrolled in this study. The median age was 63 yr (21-98), and the median follow-up duration was 44 months (12-97).
The clinical and pathological data (local), including sex, age, tumor size (<3 cm or ≥3 cm), multiplicity (single or multiple), T category (Ta or T1), tumor grade, presence of concomitant Cis or squamous differentiation, and intravesical therapy were obtained from each hospital and merged. One-thousand and eighty-two patients were treated with intravesical therapy after TUR, and drugs used for intravesical therapy were as follows: BCG in 827 patients, mitomycin-C in 108 patients, epirubicin in 135 patients, and other drugs in 12 patients. Follow-up data were also obtained, including pathologically proven recurrence and time to first recurrence, which was defined as the time period between the date of initial diagnosis and the date of recurrence. Patients who were still alive or who had died before a recurrence were censored at the date of the last available follow-up cystoscopy. Patient characteristics are summarized in Table 1.
The recurrence-free survival curve was estimated using the Kaplan-Meier method. We assessed the impact of various clinical and pathological features on time to first recurrence. Univariate and multivariate Cox proportional hazard models and logistic regression models were used. The predictive accuracy of each univariate and multivariate logistic regression model was tested using the area under the receiver operating characteristics (ROC) curve. All univariate and multivariate models were internally validated with 500 bootstrap re-samples as a means of calculating the most unbiased predictive accuracy. Statistical significance in this study was set as a p value less than 0.05. All reported p values are two-sided. All statistical tests were performed using R software (R, version 2.4.1, the R Project for Statistical Computing, http://www.r-project.org).
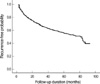
Of 1,578 patients, recurrence developed in 605 patients (38.3%). Three-year and five-year recurrence-free rates were 64.3% and 55.3%, respectively (Fig. 1). On univariate Cox proportional hazard analysis, age, tumor size, multiplicity, tumor grade, concomitant Cis, and intravesical therapy had a significant influence on recurrence-free survival (p<0.05) (Table 2).
Multivariate analysis revealed that age (hazard ratio [HR]=1.437, p<0.001), tumor size (HR=1.328, p=0.001), multiplicity (HR=1.505, p<0.001), tumor grade (HR=1.347, p=0.007), concomitant Cis (HR=1.611, p=0.007), and intravesical therapy (HR=0.681, p<0.001) were independent predictors for disease-recurrence (Table 3). Based on this analysis, nomograms for the prediction of recurrence probabilities at three and five years were constructed (Fig. 2). The scales of the nomograms reflected the coefficients from the Cox model rescaled to a user-friendly (100 point) range. The bootstrapping estimate of the predictive accuracy of the nomograms suggested that the area under the ROC curve was approximately 0.599 at three years and 0.604 at five years. The results of bootstrap re-samples were 0.723 for the three-year estimation of the nomogram and 0.738 for the five-year estimation.
The prognostic importance of various factors is not always consistent among various studies, and analyses of prognostic factors of the bladder TCC related to recurrence are sometimes difficult to compare (8). This difficulty may be due to differences in the choice of the variables analyzed, their coding, and, in multivariate analyses, the correlation among the factors (10). Another important source of variability stems from differences in tumor status, especially between primary or recurrent tumors.
This study showed that patient age, tumor size, multiplicity, tumor grade, Cis, and intravesical therapy are significant predictors for recurrence in patients with Ta, T1 TCC of the bladder. There was no difference in the time to the first recurrence between grade II and grade III tumors in the univariate analysis, so a dichotomized tumor grade (grade I vs. grade II/III) was used for the multivariate analysis (Table 2). In addition, differences among intravesical instillation agents had no influence on time to first recurrence, and intravesical therapy was also simply dichotomized (not performed vs. performed) (Table 2). Of these, multiplicity and intravesical therapy following TUR were the most influential determinants for prediction of recurrence in nomograms (Fig. 2). Tumor grade, tumor size, and patient age were less strong predictors than the ones mentioned previously. Concomitant Cis was a relatively weak variable, especially in nomograms for five years (Fig. 2).
In addition, in this study, the study cohort was limited to tumors that were newly diagnosed between 1998 and 2002. Nevertheless, tumor grade, T stage, concomitant Cis, tumor size, intravesical therapy, tumor status (primary vs. recurrent), and recurrence rate among other factors were generally identified as independent prognostic factors for disease recurrence (2-18). In some studies, age, tumor location, and sex have also been regarded as independent risk factors (15, 17, 18).
Notably, our nomograms showed that age is a strong determinant for prediction of recurrence. Younger patients appear to have a more favorable prognosis because they present more frequently with non-muscle invasive, low-grade tumors. However, studies have shown that the risk for disease progression is the same, grade-for-grade, in younger patients as in older ones (20, 21). In contrast, our results suggest that age has a prognostic impact independent from other factors. These observations are consistent with the work by Shariat et al. (22). We cannot explain why the age factor plays an important role in disease recurrence. Differences in genetic and molecular aberrations in bladder tumors and accumulation of these aberrations in older patients may relate to age. Further investigation is needed to confirm these hypotheses.
The nomograms in Fig. 2 may be useful for patient counseling because they predict the probability that the patient will encounter recurrent bladder cancer within the next three to five years. They are also effective tools for selecting patients for experimental adjuvant therapy because they are likely to be more prognostically accurate than the typical risk stratification approaches that form patient groups by placing cutoffs on variables. In the context of a randomized clinical trial, the nomograms may be useful for detecting baseline risk imbalances between treatment arms. This procedure could be accomplished by computing predicted probabilities for each patient, which would simultaneously consider all prognostic variables, and by comparing the predictions across treatment arms. In our study, we suggested two nomograms that were very similar, but there were some differences in the weight of risk score. The nomogram for three years would be more suitable for short-term studies, and the five-year nomogram may be helpful for long-term studies. Additionally, nomograms may aid in determining the scheduling of follow-up visits, as patients at lower risk for relapse or a second primary tumor may require less stringent follow-up evaluations (23).
The nomogram in Fig. 2 has several weaknesses. An important limitation to consider is that the nomogram is not entirely accurate. The prediction for the area under the ROC curve was approximately 0.599 for the three-year nomogram and was 0.604 for the five-year nomogram. Despite being imperfect, the nomogram may provide the most accurate predictions currently available. Another limitation is that the nomogram predicts disease recurrence only within a maximum period of five years. The nomogram is additionally limited because it relies on postoperative variables, making it an inadequate preoperative patient counseling tool. It is important to remember that adjuvant therapy performed in this data set was a mixture of various intravesical therapies, and the decision as to which instillation drug should be chosen was left to the physicians. Finally, experiencing a beneficial effect on intravesical therapy in this study does not mean that adjuvant intravescial therapy has to be performed in all cases of non-muscle invasive bladder tumors.
The nomograms for non-muscle invasive bladder cancer in which a point system was used are very rare. To our knowledge, only Shariat et al. (22) have reported that nomograms for non-muscle invasive bladder cancer can predict recurrence and progression, which included nuclear matrix protein 22 and cytology, as well as conventional predictors. We tested only conventional variables without ancillary markers, i.e., nuclear matrix protein 22. For this reason, the nomograms in our study may be more feasible in common clinical settings.
In conclusion, current nomograms can be used to predict the probability of disease recurrence in patients with newly diagnosed Ta, T1 transitional cell carcinoma of the bladder. They may be useful for patient counseling, clinical trial design, and patient follow-up planning.
Figures and Tables
ACKNOWLEDGEMENT
This study was sponsored by the Korean Urological Oncology Society (KUOS). We thank the other members of the KUOS, and we especially appreciate the 38 hospitals and the urologists who participated in this multicenter study.
References
1. Prout GR Jr. Management (control) of early bladder lesions. Cancer Res. 1977. 37:2891–2894.
2. Allard P, Bernard P, Fradet Y, Tetu B. The early clinical course of primary Ta and T1 bladder cancer: a proposed prognostic index. Br J Urol. 1998. 81:692–698.

3. Dalesio O, Schulman CC, Sylvester R, De Pauw M, Robinson M, Denis L, Smith P, Viggiano G. Prognostic factors in superficial bladder tumors. A study of the European Organization for Research on Treatment of Cancer: Genitourinary Tract Cancer Cooperative Group. J Urol. 1983. 129:730–733.

4. Kaasinen E, Rintala E, Hellstrom P, Viitanen J, Juusela H, Rajala P, Korhonen H, Liukkonen T. Factors explaining recurrence in patients undergoing chemoimmunotherapy regimens for frequently recurring superficial bladder carcinoma. Eur Urol. 2002. 42:167–174.

5. Kiemeney LA, Witjes JA, Heijbroek RP, Verbeek AL, Debruyne FM. Predictability of recurrent and progressive disease in individual patients with primary superficial bladder cancer. J Urol. 1993. 150:60–64.

6. Kurth KH, Denis L, Bouffioux C, Sylvester R, Debruyne FM, Pavone-Macaluso M, Oosterlinck W. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer. 1995. 31A:1840–1846.

7. Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Algaba F, Vicente-Rodriguez J. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000. 164:680–684.
8. Millan-Rodriguez F, Chechile-Toniolo G, Salvador-Bayarri J, Palou J, Vicente-Rodriguez J. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol. 2000. 163:73–78.
9. Parmar MK, Freedman LS, Hargreave TB, Tolley DA. Prognostic factors for recurrence and followup policies in the treatment of superficial bladder cancer: report from the British Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party). J Urol. 1989. 142:284–288.

10. Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, Newling DW, Kurth K. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006. 49:466–465.

11. Heney NM, Ahmed S, Flanagan MJ, Frable W, Corder MP, Hafermann MD, Hawkins IR. Superficial bladder cancer: progression and recurrence. J Urol. 1983. 130:1083–1086.

12. Kiemeney LA, Witjes JA, Heijbroek RP, Koper NP, Verbeek AL, Debruyne FM. Should random urothelial biopsies be taken from patients with primary superficial bladder cancer? A decision analysis. Members of the Dutch South-East Co-Operative Urological Group. Br J Urol. 1994. 73:164–171.
13. Loening S, Narayana A, Yoder L, Slymen D, Penick G, Culp D. Analysis of bladder tumor recurrence in 178 patients. Urology. 1980. 16:137–141.

14. Mulders PF, Meyden AP, Doesburg WH, Oosterhof GO, Debruyne FM. Prognostic factors in pTa-pT1 superficial bladder tumours treated with intravesical instillations. The Dutch South-Eastern Urological Collaborative Group. Br J Urol. 1994. 73:403–408.
15. Narayana AS, Loening SA, Slymen DJ, Culp DA. Bladder cancer: factors affecting survival. J Urol. 1983. 130:56–60.

16. Pawinski A, Sylvester R, Kurth KH, Bouffioux C, van der Meijden A, Parmar MK, Bijnens L. A combined analysis of European Organization for Research and Treatment of Cancer, and Medical Research Council randomized clinical trials for the prophylactic treatment of stage TaT1 bladder cancer. European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council Working Party on Superficial Bladder Cancer. J Urol. 1996. 156:1934–1940.
17. Shinka T, Matsumoto M, Ogura H, Hirano A, Ohkawa T. Recurrence of primary superficial bladder cancer treated with prophylactic intravesical Tokyo 172 bacillus Calmette-Guerin: a long-term follow-up. Int J Urol. 1997. 4:139–143.

18. Witjes JA, Kiemeney LA, Schaafsma HE, Debruyn FM. The influence of review pathology on study outcome of a randomized multicentre superficial bladder cancer trial. Members of the Dutch South East Cooperative Urological Group. Br J Urol. 1994. 73:172–176.
19. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC Cancer Staging Manual. 2002. 6th ed. New York: Springer Verlag;Pages.
20. Wan J, Grossman HB. Bladder carcinoma in patients age 40 years or younger. Cancer. 1989. 64:178–181.

21. Linn JF, Sesterhenn I, Mostofi FK, Schoenberg M. The molecular characteristics of bladder cancer in young patients. J Urol. 1998. 159:1493–1496.

22. Shariat SF, Zippe C, Ludecke G, Boman H, Sanchez-Carbayo M, Casella R, Mian C, Friedrich MG, Eissa S, Akaza H, Sawczuk I, Serretta V, Huland H, Hedelin H, Rupesh R, Miyanaga N, Sagalowsky AI, Wians F Jr, Roehrborn CG, Lotan Y, Perrotte P, Benayoun S, Marberger MJ, Karakiewicz PI. Nomograms including nuclear matrix protein 22 for prediction of disease recurrence and progression in patients with Ta, T1 or CIS transitional cell carcinoma of the bladder. J Urol. 2005. 173:1518–1525.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download