Abstract
This study aimed to investigate the relationship of caveolin-1 expression with prognosis in patients with transitional cell carcinoma of the upper urinary tract (TCC-UUT). Formalin-fixed, paraffin-embedded tissue sections of TCC-UUT from 98 patients, who had undergone radical nephroureterectomy, were stained immunohistochemically using antibodies against caveolin-1. The expression pattern of caveolin-1 was compared with the clinicopathological variables. The caveolin-1 expression was significantly correlated with T stage (p<0.001) and grade (p=0.036). The survival rate of patients with caveolin-1 positive tumors was significantly lower than that of patients with caveolin-1 negative tumors (p<0.0001). The univariate analyses identified T stage, grade, and caveolin-1 expression as significant prognostic factors for cancer-specific survival, whereas the multivariate analyses indicated that T stage and caveolin-1 expression were independent prognostic factors. These results show that the increased expression of caveolin-1 is associated with tumor progression and poor prognosis in TCC-UUT, suggesting that caveolin-1 may play an important role in the progression of TCC-UUT.
Transitional cell carcinoma of the upper urinary tract (TCC-UUT) is a relatively uncommon disease, accounting for 4.5-9% of all renal tumors and 5-6% of all urothelial tumors (1). The incidence of TCC-UUT appears to have increased recently, probably due to increased environmental exposure and aging population (2). The upper urinary tract has anatomical characteristics such as a thin muscle layer, proximity to the kidney and rich lymphatic drainage. Tumor invasion may significantly influence distant metastasis and progression in patients with TCC-UUT (3). However, the exact molecular mechanisms of tumor invasion, recurrence, progression, and prognosis of this disease remain largely unclear.
Caveolins are the major structural proteins of caveolae, the vesicular invaginations of the plasma membrane. The caveolin family includes caveolin-1, -2, and -3. Caveolin-1 and -2 are co-expressed in many cell types including adipocytes, endothelial cells, smooth muscle cells, and fibroblasts, whereas the expression of caveolin-3 is muscle-specific (4-6). Caveolin-1 has been implicated in intracellular transport, membrane trafficking, and signal transduction (5), and has also been shown to play an important role in various human pathological conditions, including cancer, diabetes, bladder dysfunction, muscular dystrophy, and those related to the cardiovascular system such as atherosclerosis, cardiac hypertrophy, cardiomyopathy, pulmonary hypertension, and neointimal hyperplasia (6, 7).
The role of caveolin-1 in cancers remains controversial, because caveolin-1 has been reported to be dysregulated in various cancers, but the pattern of dysregulation appears to vary with cancer types. The caveolin-1 expression is decreased in several cancers such as ovarian carcinoma, pulmonary adenocarcinoma, various soft tissue sarcomas, and breast cancer and thought to function as a tumor suppressor gene (8-11). On the other hand, the caveolin-1 expression is upregulated in the other cancers such as renal cell carcinoma, prostate cancer, bladder cancer, colonic adenocarcinoma, and esophageal squamous cell carcinoma and associated with higher stage, cancer progression, and poor prognosis (12-17).
There have been no previous studies about caveolin-1 expression in TCC-UUT. In the present study, therefore, we investigated the relationship between caveolin-1 expression and prognosis in patients with TCC-UUT.
Formalin-fixed, paraffin-embedded, archival surgical specimens that had been obtained from 98 patients (76 men and 22 women; mean age of 61.7 yr, range 33-85 yr), who had been diagnosed with TCC-UUT, were assessed. All patients had undergone radical nephroureterectomy either at Ajou University Hospital or Yonsei Medical Center between November 1994 and December 2004. Tumors were staged using the 2002 TNM staging system (18) and graded according to the World Health Organization (WHO)/International Society of Urological Pathology (ISUP) grading criteria (19).
Immunohistochemical staining for the expression of caveolin-1 was carried out as previously described (12). Briefly, paraffin-embedded 4- m tissue sections were deparaffinized, treated with 3% hydrogen peroxide, followed by incubation with the blocking reagent, and then incubated with mouse monoclonal antibody against caveolin-1 (clone 2297, diluted 1:200; BD Biosciences, San Diego, CA, U.S.A.) for 1 hr at 37℃. Staining was carried out by the standard streptavidin-biotin-peroxidase complex method. Positive caveolin-1 staining of smooth muscle cells or endothelium, known to be abundant in caveolin-1, served as an internal positive control. Negative controls were obtained by omitting the primary antibody.
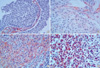
The immunostaining was independently evaluated by two pathologists who were unaware of the clinical data. The caveolin-1 expression was based on the presence of cytoplasmic and/or membranous staining, which was semi-quantitatively estimated according to the methods described by Sinicrope et al. (20), with minor modifications. On the basis of the percentages of immunopositive cells, the data were subdivided into five categories: 0, ≤10%; 1, 11-25%; 2, 26-50%; 3, 51-75%; and 4, >75% positive cells. The immunointensity was also subclassified into four categories: 0, negative; 1, weak; 2, moderate (same intensity of smooth muscle cells); and 3, strong (Fig. 1). The immunoreactive score (caveolin-1 expression) for each case was generated by multiplying the values for the two parameters. A score of 0 was considered as negative, while all other scores were considered as positive.
The correlation between caveolin-1 expression and various clinicopathological variables was analyzed by the chi-square test. The cancer-specific survival calculations were illustrated with Kaplan-Meier curves, and univariate and multivariate analyses were performed using the log-rank test and the Cox proportional hazards regression model, respectively. The values of p<0.05 were considered to be statistically significant in all of the analyses. All statistical analyses were performed using SPSS®, version 12.0.
The clinicopathological characteristics of the 98 patients with TCC-UUT are summarized in Table 1. There were no distant metastases in any patients at the time of radical nephroureterectomy. Seventy patients were disease-free at a median follow-up of 52.5 months (range, 12-162 months). The other 28 patients developed metastases at a median of 28 months (range, 4-86 months) after radical nephroureterectomy. Twenty-seven patients died of disease during the follow-up period.
The patterns of caveolin-1 expression in the 98 patients are summarized in Table 2. Of the 98 sections, positive immunostaining for caveolin-1 was observed in 10 sections (10.2%).
The caveolin-1 expression was significantly correlated with T stage (p<0.001) and grade (p=0.036), but not with N stage (p=0.149), tumor location (p=0.847), previous history of bladder cancer (p=0.711), coexisting bladder cancer at diagnosis (p=0.211), or bladder cancer recurrence during the follow-up (p=0.691) (Table 3).
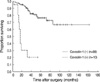
A Kaplan-Meier survival curve showed that the survival rate of patients with caveolin-1 positive tumors was significantly lower than that of patients with caveolin-1 negative tumors (p<0.0001) (Fig. 2).
The univariate analyses identified T stage (p<0.0001), grade (p=0.0172), and caveolin-1 expression (p<0.0001) as significant prognostic factors for cancer-specific survival, whereas the multivariate analyses indicated that T stage (p=0.036) and caveolin-1 expression (p=0.002) were independent prognostic factors (Table 4).
The role of caveolin-1 in cancers still remains controversial. Several studies have suggested that caveolin-1 could function as a tumor suppressor gene. The genes encoding caveolin-1 and -2 are localized at the D7S522 locus located on human chromosome 7q31.1, a region frequently deleted in various types of human cancers (21). The allelic loss at chromosome 7q31.3 occurred in all grades and stages of invasive ovarian carcinomas, but not in borderline ovarian tumors, suggesting that the inactivation of a tumor suppressor gene in this region is an early event in ovarian tumorigenesis (22). Caveolin-1 (-/-) null mice showed acceleration of the development of dysplastic mammary lesions (23), and caveolin-1 transfected human breast cancer cell line MCF-7 cells showed less proliferation than the vector control transfectants (11). In addition, the caveolin-1 expression has been found to be down-regulated in ovarian carcinoma, pulmonary adenocarcinoma, and sarcomas (8-10). Taken together, these findings suggest that caveolin-1 has tumor suppressor characteristics.
In contrast, however, several studies have shown elevated caveolin-1 expression levels in some cancers and cancer cell lines. The increased expression of caveolin-1 has been found to be associated with drug-resistant cancer cell lines, such as paclitaxel or etoposide-resistant human lung cancer cells and androgen-insensitive mouse prostate cancer cells (24-26). Furthermore, the increased caveolin-1 expression has been reported in renal cell carcinoma, prostate cancer, bladder cancer, colonic carcinoma, and esophageal squamous cell carcinoma. In many of these tumors, caveolin-1 overexpression was associated with higher tumor stage, tumor invasion, tumor size, higher grade, and poor prognosis (12-17). These findings indicate that caveolin-1 may function as a tumor promoter during carcinogenesis rather than as a tumor suppressor.
The differential expression of caveolin-1, depending on the histologic subtypes of cancers or different malignant stages, has also been reported. In the majority of small cell lung cancers, caveolin-1 expression was lost through promoter methylation, the major mode of inactivation of many tumor suppressor genes in human cancers, whereas caveolin-1 expression in the majority of non-small cell lung cancers was retained and appeared to be required for tumor growth through the activation of FAK and Ra1A signaling (27). In lung adenocarcinomas, the caveolin-1 served as a tumor suppressor with the loss of caveolin-1 regulation, resulting in tumor extension and dedifferentiation, whereas caveolin-1 overexpression in lung squamous cell carcinoma was correlated with tumor extension, suggesting that these reciprocal functions of caveolin-1 are due to different activation states of the different domains of caveolin-1 and altered interactions with binding partners (28). In oral squamous cell carcinomas, the rate of caveolin-1 immunoreactivity increased progressively from normal oral mucosa, oral pre-cancer lesions to primary carcinomas, whereas it decreased from primary to metastatic carcinomas. These findings indicated the possibility of biphasic behavior of caveolin-1 in oral carcinogenesis and metastasis (29). Therefore, caveolin-1 might function either as a tumor promoter or as a tumor suppressor, depending on the tumor cell types or malignant stages.
For the expression of caveolin-1 in urothelial carcinomas, studies have been done in bladder carcinomas, but not in TCC-UUT. Rajjayabun et al. (14) found that the caveolin-1 expression was correlated with tumor stage and grade of bladder TCC, but not with tumor multiplicity, recurrence, progression, or survival of patients. Positive immunostaining for caveolin-1 was detected in 10.1% of tumor specimens. Fong et al. (15) showed that positive caveolin-1 immunostaining was detected in 37% of urothelial carcinoma, but not in nonneoplastic urothelium, and that the elevated expression of caveolin-1 and -2 was correlated with tumor grade and squamous differentiation.
In the present study, we investigated the expression of caveolin-1 in TCC-UUT and found that the positive immunostaining for caveolin-1 was observed in 10.2% of sections, similar to the findings of Rajjayabun et al. (14) in bladder TCC. Furthermore, the caveolin-1 expression was correlated significantly with T stage and grade. The survival rate of patients with caveolin-1 positive tumors was significantly lower than that of patients with caveolin-1 negative tumors. Also, the caveolin-1 expression was an independent prognostic factor for cancer-specific survival. These findings strongly indicate that caveolin-1 is involved in tumor progression, and that the increased expression of caveolin-1 is a late event in urothelial carcinomas.
In conclusion, the increased expression of caveolin-1 is associated with tumor progression and poor prognosis in TCC-UUT, suggesting that caveolin-1 may play an important role in the progression of TCC-UUT.
Figures and Tables
Fig. 1
Immunohistochemical staining for caveolin-1. (A) Cancer cells were not stained (caveolin-1 intensity 0). (B) Smooth muscle cells were stained as an internal control. Cancer cells were weakly stained, compared to an internal control (caveolin-1 intensity 1). (C) Cancer cells were stained in same intensity as internal control (caveolin-1 intensity 2). (D) Cytoplasms of cancer cells were strongly stained for caveolin-1 (caveolin-1 intensity 3). Original magnification, ×400.
References
1. Iborra I, Solsona E, Casanova J, Ricos JV, Rubio J, Climent MA. Conservative elective treatment of upper urinary tract tumors: a multivariate analysis of prognostic factors for recurrence and progression. J Urol. 2003. 169:82–85.

2. Munoz JJ, Ellison LM. Upper tract urothelial neoplasms: incidence and survival during the last 2 decades. J Urol. 2000. 164:1523–1525.

3. Miyata Y, Kanda S, Nomata K, Eguchi J, Kanetake H. Expression of cyclooxygenase-2 and EP4 receptor in transitional cell carcinoma of the upper urinary tract. J Urol. 2005. 173:56–60.

4. Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing "preassembled signaling complexes" at the plasma membrane. J Biol Chem. 1998. 273:5419–5422.

5. Liu P, Rudick M, Anderson RG. Multiple functions of caveolin-1. J Biol Chem. 2002. 277:41295–41298.

6. Williams TM, Lisanti MP. The caveolin genes: from cell biology to medicine. Ann Med. 2004. 36:584–595.

7. Schwencke C, Braun-Dullaeus RC, Wunderlich C, Strasser RH. Caveolae and caveolin in transmembrane signaling: implications for human disease. Cardiovasc Res. 2006. 70:42–49.

8. Wiechen K, Diatchenko L, Agoulnik A, Scharff KM, Schober H, Arlt K, Zhumabayeva B, Siebert PD, Dietel M, Schäfer R, Sers C. Caveolin-1 is down-regulated in human ovarian carcinoma and acts as a candidate tumor suppressor gene. Am J Pathol. 2001. 159:1635–1643.

9. Wikman H, Kettunen E, Seppänen JK, Karjalainen A, Hollmen J, Anttila S, Knuutila S. Identification of differentially expressed genes in pulmonary adenocarcinoma by using cDNA array. Oncogene. 2002. 21:5804–5813.

10. Wiechen K, Sers C, Agoulnik A, Arlt K, Dietel M, Schlag PM, Schneider U. Down-regulation of caveolin-1, a candidate tumor suppressor gene, in sarcomas. Am J Pathol. 2001. 158:833–839.

11. Hino M, Doihara H, Kobayashi K, Aoe M, Shimizu N. Caveolin-1 as tumor suppressor gene in breast cancer. Surg Today. 2003. 33:486–490.

12. Joo HJ, Oh DK, Kim YS, Lee KB, Kim SJ. Increased expression of caveolin-1 and microvessel density correlates with metastasis and poor prognosis in clear cell renal cell carcinoma. BJU Int. 2004. 93:291–296.

13. Yang G, Truong LD, Wheeler TM, Thompson TC. Caveolin-1 expression in clinically confined human prostate cancer: a novel prognostic marker. Cancer Res. 1999. 59:5719–5723.
14. Rajjayabun PH, Garg S, Durkan GC, Charlton R, Robinson MC, Mellon JK. Caveolin-1 expression is associated with high-grade bladder cancer. Urology. 2001. 58:811–814.

15. Fong A, Garcia E, Gwynn L, Lisanti MP, Fazzari MJ, Li M. Expression of caveolin-1 and caveolin-2 in urothelial carcinoma of the urinary bladder correlates with tumor grade and squamous differentiation. Am J Clin Pathol. 2003. 120:93–100.

16. Fine SW, Lisanti MP, Galbiati F, Li M. Elevated expression of caveolin-1 in adenocarcinoma of the colon. Am J Clin Pathol. 2001. 115:719–724.

17. Kato K, Hida Y, Miyamoto M, Hashida H, Shinohara T, Itoh T, Okushiba S, Kondo S, Katoh H. Overexpression of caveolin-1 in esophageal squamous cell carcinoma correlates with lymph node metastasis and pathologic stage. Cancer. 2002. 94:929–933.

18. Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. AJCC cancer staging manual. 2002. 6th ed. New York: Springer-Verlag;329–331.
19. Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998. 22:1435–1448.
20. Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995. 55:237–241.
21. Engelman JA, Zhang XL, Lisanti MP. Genes encoding human caveolin-1 and -2 are co-localized to the D7S522 locus (7q31.1), a known fragile site (FRA7G) that is frequently deleted in human cancers. FEBS Lett. 1998. 436:403–410.

22. Edelson MI, Scherer SW, Tsui LC, Welch WR, Bell DA, Berkowitz RS, Mok SC. Identification of a 1300 kilobase deletion unit on chromosome 7q31.3 in invasive epithelial ovarian carcinomas. Oncogene. 1997. 14:2979–2984.

23. Williams TM, Cheung MW, Park DS, Razani B, Cohen AW, Muller WJ, Di Vizio D, Chopra NG, Pestell RG, Lisanti MP. Loss of caveolin-1 gene expression accelerates the development of dysplastic mammary lesions in tumor-prone transgenic mice. Mol Biol Cell. 2003. 14:1027–1042.

24. Yang CP, Galbiati F, Volonte D, Horwitz SB, Lisanti MP. Upregulation of caveolin-1 and caveolae organelles in Taxol-resistant A549 cells. FEBS Lett. 1998. 439:368–372.

25. Belanger MM, Gaudreau M, Roussel E, Couet J. Role of caveolin-1 in etoposide resistance development in A549 lung cancer cells. Cancer Biol Ther. 2004. 3:954–959.
26. Nasu Y, Timme TL, Yang G, Bangma CH, Li L, Ren C, Park SH, DeLeon M, Wang J, Thompson TC. Suppression of caveolin expression induces androgen sensitivity in metastatic androgen-insensitive mouse prostate cancer cells. Nat Med. 1998. 4:1062–1064.

27. Sunaga N, Miyajima K, Suzuki M, Sato M, White MA, Ramirez RD, Shay JW, Gazdar AF, Minna JD. Different roles for caveolin-1 in the development of non-small cell lung cancer versus small cell lung cancer. Cancer Res. 2004. 64:4277–4285.





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download