Abstract
Granulocyte macrophage-colony stimulating factor (GM-CSF) has immuno-stimulatory effects. We hypothesized that GM-CSF plays an important role both in lipopolysaccharide (LPS)- and hemorrhage-induced acute lung injury (ALI). We also postulated that GM-CSF augments LPS-induced inflammation by priming neutrophils. ALI was induced in GM-CSF-/- or control C57BL mice either by LPS injection or by hemorrhage. Lung inflammation (by lung expression for tumor necrosis factor-α (TNF-α), macrophage inflammatory protein-2 (MIP-2), interleukin-1β (IL-1β), interleukin-6 (IL-6), and keratinocyte-derived chemokine) and lung injury (by myeloperoxidase and evans blue dye assay) were evaluated after ALI. Incremental doses of LPS (0, 1, 10, and 100 ng/mL) and GM-CSF (0, 1, 10, and 100 ng/mL) were added to bone marrow neutrophils. The expression of TNF-α, MIP-2, and IL-1β was evaluated with enzyme linked immunosorbent assay. The mRNA expression of three cytokines, and the nuclear translocation of nuclear factor kappa B (NFκ-B) were evaluated by reverse transcriptase-polymerase chain reaction and electropnoretic mobility shift assay, respectively. GM-CSF -/- mice showed decreased neutrophil infiltration, less leakage, and lower expression of cytokines in the lung after LPS or hemorrhage. GM-CSF augmented LPS-induced protein and mRNA expression of TNF-α, MIP-2 and IL-1β, which was mediated by increased intra-nuclear translocation of NF-κB. GM-CSF plays an important role in high-dose LPS and hemorrhage-induced ALI, which appears to be mediated by its priming effect on neutrophils.
Granulocyte macrophage-colony stimulating factor (GM-CSF) is a glycoprotein of 22 kDa. It is synthesized and secreted by a wide variety of activated cells including: T lymphocytes, macrophages, lung epithelial cells, and cytokine-activated endothelial cells (1, 2). GM-CSF plays an important role in promoting the growth and differentiation of myelomonocytic progenitors (3, 4). The immuno-stimulatory effects of GM-CSF have been widely applicated in clinical practice. For example, febrile, neutropenic patients after chemotherapy show significant increase in total white blood cell and absolute neutrophil counts after GM-CSF treatment (5). On the other hand, GM-CSF can enhance the functional responsiveness and extend the life of mature polymorphonuclear leukocytes (6). Because neutrophils play a key role in systemic inflammation, unnecessary prolongation of the life span of granulocytes may augment tissue injury. As expected, administration of GM-CSF has been associated with side-effects such as shock, hypoxia, liver dysfunction, and acute lung injury (7, 8). In this sense, immuno-stimulatory effects of GM-CSF can be beneficial or harmful depending on the clinical situation.
Acute lung injury (ALI) is a syndrome of severe dyspnea, hypoxemia, and diffuse pulmonary infiltrates leading to respiratory failure. ALI is caused by many disorders that induce either direct or indirect type of lung injury. Although sepsis is one of the most common causes of ALI, blood loss is also frequently associated with lung injury. For example, loss of approximately 30% of total blood volume is an important threshold predicting the subsequent development of ALI and other organ system dysfunction (9). Although GM-CSF has frequently been reported to participate in the development of sepsis-induced lung injury, there has been no study evaluating the role of GM-CSF in hemorrhage-induced ALI.
In the present study, we hypothesized that GM-CSF plays an important role not only in lipopolysaccharide (LPS)-induced but also in hemorrhage-induced ALI. We also speculated that GM-CSF augments LPS-induced inflammation by its priming effect on neutrophils.
Male Balb/c mice, 8-12 week of age, were purchased from Harlan Sprague Dawley (Indianapolis, IN, U.S.A.). The mice were kept on a 12:12-hr light-dark cycle with free access to food and water. All experiments were conducted in accordance with institutional review board-approved protocols. GM-CSF knockout mice were kindly donated by Dr. Dranoff (Dana-Farber Cancer Institute, Boston, MA, U.S.A.), who established and investigated mice carrying a null allele of the GM-CSF gene (10). C57BL mice were purchased from Jackson Laboratory (Bar Harbor, ME, U.S.A.) and used as wild-type controls.
Both mice (GM-CSF -/- and control C57BL) received an intraperitoneal injection of LPS at a dose of 0.5 mg/kg or 5 mg/kg in 0.2 mL phosphate buttered saline (PBS). Mice were anesthesized 4 hr after LPS injection and chest was opened and flushed by infusing 10 mL of PBS into beating right ventricle. After then, lungs were removed and stored at -70℃ before being used for cytokines and myeloperoxidase (MPO) assay.
The mouse hemorrhage model used in these experiments has been described previously (11). In brief, mice were anesthesized with inhaled isofluorane. Cardiac puncture was used to remove 30% of the calculated total blood volume (0.27 mL/10 g body wt) over 60 sec into a heparanized syringe. One hour after the induction of hemorrhage, mice were again anesthesized with isofluorane, and the previously removed blood was infused into the retro-orbital venous plexus. The sham procedure involved cardiac puncture under isofluorane anesthesia, without blood removal, followed by a second episode of anesthesia and retro-orbital puncture 1 hr later.
Lung samples were homogenized in ice cold lysis buffer (50 mM HEPES, 150 mM NaCl, 10% glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA, 1 mM sodium vanadate, 10 mM sodium pyrophosphate, 10 mM NaF, 300 µM p-nitrophenyl phosphate, 1 mM PMSF, 10 µg/mL leupeptin, 10 µg/mL aprotinin, pH 7.3) containing 1 mM of protease inhibitor cocktail (Sigma, St Louis, MO, U.S.A.). Homogenates were centrifuged at 14,000 g for 15 min and supernatant was collected. The protein concentration of each sample was assayed using the micro BCA protein assay kit standardized to bovine serum albumin (BSA), according to manufacturer's protocol (Pierce, Rockford, IL, U.S.A.).
MPO activity was assayed as reported previously with minor modifications (11). Lung tissue was homogenized in 1.0 mL of 50 mM potassium phosphate buffer (pH 6.0) containing 10 mM N-ethylmaleimide for 30 sec on ice. The homogenate was centrifuged at 12,000 g for 30 min at 4℃. The proteinous pellet was homogenized once more in ice-cold buffer, and the homogenate was centrifuged (12,000 g for 30 min at 4℃). The pellet was resuspended and sonicated on ice for 90 sec in 10 times vol of hexadecyltrimethylammonium bromide buffer (0.5% hexadecyltrimethylammonium in 50 mM potassium phosphate, pH 6.0). Samples were incubated in a water bath (56℃) for 2 hr and then centrifuged at 12,000 g for 10 min. The supernatant was collected for assay of MPO activity as determined by measuring the H2O2-dependent oxidation of o-DA (3,3'-dimethoxy-benzidine dihydrochloride) at 460 nm.
Evans blue dye (EBD) assay was performed to assess lung leak using separate animals. EBD solution (5 mg/mL, 200 µL) was injected through a tail vein (50 mg/kg). One hour later, animals were anesthetized with inhaled isofluorane and the chest was opened. The pulmonary vasculature was flushed free of blood by gentle infusion of 10 mL of PBS into the beating right ventricle. The lungs were then excised, weighed and dried at 60℃ for 24 hr, then placed in 3 mL of formamide at 60℃ for 36 hr to extract EBD. Dye content was evaluated by spectrophotometry at 620 nm.
Isoflurane was obtained from Abbott Laboratories (Chicago, IL, U.S.A.). RPMI 1640/25 mM HEPES/L-glutamine was obtained from Bio-Whittaker Products (Walkersville, MD, U.S.A.), and fetal bovine serum (FBS) and penicillin/streptomycin were purchased from Gemini Bioproducts (Calabasas, CA, U.S.A.). Custom cocktail antibodies and columns for neutrophil isolation were purchased from Stem Cell Technologies (Vancouver, BC, Canada). All other reagents were purchased from Sigma unless otherwise noted in the text.
Bone marrow neutrophils were isolated according to the manufacturer's instructions. To obtain the bone marrow cell suspension, the femur and tibia of a mouse were flushed with RPMI 1640. Tissue fragments were removed by rapid filtration through a glass wool column, and cells were collected by centrifugation. The cell pellets were resuspended in RPMI 1640, 1% fetal calf serum (FCS) and then incubated with primary antibodies specific for cell surface markers F4/80, CD4, CD45R, CD5, and TER119 for 15 min at 4℃. This custom mixture is specific for T and B cells, RBC, monocytes, and macrophages. After 15-min incubation, 100 µL of antibiotin tetrameric antibody complexes were added and the cells were incubated for 15 min at 4℃. After this, 60 µL of colloidal magnetic dextran iron particles were added to the suspension and incubated for 15 min at 4℃. The entire cell suspension was then placed into a column surrounded by a magnet. The T cells, B cells, RBC, monocytes, and macrophages were captured in the column, allowing the neutrophils to pass through by negative-selection methods. Neutrophil purity, as determined by Wright's stained cytospin preparations, was greater than 97%.
Neutrophils were resuspended in RPMI 1640/10%-defined fetal calf serum at a final concentration of 5×105 cells/mL and allocated into 1.5 mL microtube and stimulated with LPS (1, 10, and 100 ng/mL) with or without GM-CSF (1, 10, and 100 ng/mL) and suspended on the water bath (37℃) for 4 hr. The supernatant was collected by centrifuging the culture tube (12,000 g, 5 min) then stored at -70℃ before being used for the measurement of cytokines. For the measurement of mRNA expression, neutrophils (5×106 cells/tube) were stimulated by LPS (100 ng/mL) with or without GM-CSF (1, 10, and 100 ng/mL) on the water bath (37℃) for 1 hr.
Immunoreactive tumor necrosis factor-α (TNF-α), macrophage inflammatory protein-2 (MIP-2), interleukin-1β (IL-1β), interleukin-6 (IL-6), and keratinocyte-derived chemokine (KC) were quantitated using commercially available ELISA kits (R&D Systems, Minneapolis, MN, U.S.A.), according to the manufacturer's instructions.
RNA was isolated using the RNAeasy kit (Qiagen, Valencia, CA, U.S.A.) following the manufacturer's protocol. Primers and probes for TNF-α were designed using Primer Express software supplied by Applied Biosystems (Foster City, CA, U.S.A.). The TNF-α primer and probe consisted of the following: forward primer, 5'-CTGTAGCCCACGTCGTAGTCAA-3'; reverse primer, 5'-CTCCTGGTATGAGATAGCAAATCG-3'; probe, 6FAMTGCCCCGACTACGTGCTCCTCACMRA. The MIP-2 primer and probe consisted of the following: forward primer, 5'-TGTGACGCCCCCAGGATGTGACGCCCCCAGGA; reverse primer, 5'-AACTTTTTGACCGCCCTTGAG-3'; probe, 6FAMTGCGCCCAGACAGAAGTCATAGCCATAMRA. The IL- 1β primer and probe consisted of the following: forward primer, 5'-GCTGAAAGCTCTCCACCTCAA; reverse primer, 5'-TCGTTGCTTGGTTCTCCTTGTA; probe, 6FAMCAGAATATCAACCAACAAGTGATATTCTCCATGAGCTAMRA. All reagents used in the one-step RT-PCR were purchased from Perkin Elmer (Foster City, CA, U.S.A.). Each one-step reverse trans criptase-polymerase chain reaction (RT-PCR) contained a total volume of 50 µL. The reverse transcription reaction was performed for 30 min at 48℃ using MultiScribe Reverse Transcriptase with a final concentration of 0.25 U/µL. After the reverse transcription step, AmpliTaq Gold polymerase, with a final concentration of 0.025 U/µL, was activated by an increase in temperature to 95℃ for 10 min followed by 40 cycles of amplification (95℃ for 15 sec and 60℃ for 1 min) with a Gene Amp 5700 Sequence Detection System (Applied Biosystems, Foster City). Quantification was determined by dividing the amount of GAPDH RNA by the target amount for each cytokine RNA samples.
Nuclear extracts (20 µg) were incubated at room temperature for 15 min in 20 µL of reaction buffer containing 10 mM Tris · HCl (pH 7.5), 1 mM MgCl2, 0.5 mM EDTA, 0.5 mM dithiothreitol, 10 mM 50 mM NaCl, and 4% glycerol with 32P-end-labeled, double-stranded oligonucleotide probe specific for the B site, 5'-GCCATGGGGGGATCCCCGAAGTCC-3'(Active Motive, Carlsbad, CA, U.S.A.) and 1 µg of poly (dI-dC) · poly(dI-dC). The complexes were resolved on 5% polyacrylamide gels in Tris · HCl (pH 8.0)- borate-EDTA buffer at 10 V/cm. Dried gels were exposed with Kodak Biomax MS film (Rochester, NY, U.S.A.) for 1-24 hr at -70℃.
To limit variability and to provide appropriate controls for each experimental condition, the entire group of animals was prepared and studied at the same time. More than 5 mice were used in each group for lung cytokines, MPO, and EBD assays. One way analysis of variance (ANOVA) and Tukey-Kramer multiple comparisons test were used for analysis of multiple groups.
Both for low (0.5 mg/kg) and high doses (5 mg/kg) of intraperitoneal LPS injection, GM-CSF -/- mice showed less of an increase in MPO activity than did the C57BL control mice (p<0.05) (Fig. 1A). GM-CSF -/- mice were resistant to lung leakage even with high doses of LPS injection (Fig. 2A). GM-CSF -/- mice did not show an increase in MPO activity or lung leakage after hemorrhage (Fig. 1B, 2B).
The expression of lung TNF-α did not increase even with high doses of the LPS injection (5 mg/kg) in the GM-CSF -/- mice (Fig. 3). The expression of MIP-2 and IL-1β in the lung increased with low (0.5 mg/kg) or high (5 mg/kg) doses of LPS injection in the GM-CSF -/- mice. However, it was much lower in the GM -/- mice than in the C57BL mice (Fig. 3). The expression of lung TNF-α and MIP-2 did not increase in the hemorrhage model. Therefore, we evaluated two other cytokines, KC and IL-6. In the control state, there was no significant difference in the expression of the three cytokines (KC, IL-6, and IL-1β) between the GM-CSF -/- and the C57BL mice. However, after hemorrhage, the expression of the three cytokines in the lung was higher in the C57BL mice than in the GM-CSF -/- mice (Fig. 4).
GM-CSF showed a limited stimulatory effect on the expression of TNF-α and MIP-2 of polymorphonuclear leukocyte (PMN) when it was administered alone or with low or medium doses of LPS (LPS 1 and 10 ng/mL) (Fig. 5A, B). When incremental doses of GM-CSF (0, 1, 10, or 100 ng/mL) were added to high doses of LPS (100 ng/mL), the expression of TNF-α (mean±SEM) (468±59, 673±140, 1,264±210, and 1,916±599 pg/mL, respectively) (p<0.05) (Fig. 5A) and MIP-2 (313±64, 325±30, 569±112, and 468±74 pg/mL, respectively) (p<0.05) (Fig. 5B) of PMN increased in a dose-dependent manner. GM-CSF augmented LPS induced IL-1β expression of PMN at all three doses of administered LPS (Fig. 5C).
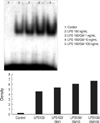
The mRNA expression of TNF-α, MIP-2, and IL-1β was higher when incremental doses of GM-CSF (1, 10, and 100 ng/mL) were added with LPS (100 ng/mL) than when LPS was used for treatment alone (p<0.05) (Fig. 6). This apparent synergistic effect of GM-CSF on LPS induced mRNA expression of three cytokines of PMN was mediated by increased intranuclear translocation of NF-κB (Fig. 7).
The immuno-stimulatory effects of GM-CSF have been utilized in clinical practice to stimulate the production of granulocytes and macrophages in neutropenic, febrile patients. In addition, because low-dose GM-CSF has been shown to be associated with improved pulmonary gas exchange, without neutrophil infiltration (12), there have been clinical trials that have evaluated GM-CSF as supportive therapy for systemic inflammatory diseases. For example, one study showed that the addition of GM-CSF to antibiotic therapy reduced the rate of infectious complications, the length of hospitalization, as well as costs, in patients with nontraumatic abdominal sepsis (13).
However, GM-CSF-induced hyper-stimulation of inflammatory cells has resulted in abnormal physiologic side effects as well. Hypothermia and loss of body weight were markedly attenuated in LPS-treated GM-CSF-deficient mice compared to similarly treated control mice (8). Moreover, the levels of circulating IFN-γ, IL-1α , and IL-6 were lower in LPS-treated GM-CSF-deficient mice than LPS-treated control mice (14). These observations suggest that GM-CSF augments the cytokine production related to LPS-mediated septic shock, and may aggravate systemic inflammation and tissue injury.
Taking into consideration of mutually contradicting findings, we hypothesized that the physiologic effects of GM-CSF on inflammation are dependent on the levels of LPS it encounters. We treated low (0.5 mg/kg) or high (5 mg/kg) doses of LPS into peritoneum of Balb/c mice to induce endotoxemia and ALI, the doses of which were in accordance with the previous study (15). Our hypothesis was supported by the in vivo study. In LPS-induced lung injury, the GM-CSF -/- mice showed less infiltration of neutrophils in the lung (in MPO assay, Fig. 1A) and more resistance to lung leakage (in EBD assay, Fig. 2A) than did the control C57BL mice. This apparent protective effect of the GM-CSF knockout state was more prominent when the dose of injected LPS was high (5 mg/kg). In addition, the expression of MIP-2 or IL-1β in the lung was lower in the GM-CSF -/- mice both at low (0.5 mg/kg) and high doses (5 mg/kg) of LPS injection (Fig. 3). Of note was that the TNF-α expression, which was not different between the two groups of mice at low doses of LPS injection, increased only in the C57BL mice with high doses of LPS injection. In the hemorrhage model, the GM-CSF -/- mice were more resistant to neutrophil infiltration (Fig. 1B) and lung leakage (Fig. 2B) than the C57BL mice after hemorrhage. In addition, the GM-CSF -/- mice showed a lower level of inflammatory cytokine (KC, IL-6, and IL-1β) expression compared to the control C57BL mice (Fig. 4). These findings imply that GM-CSF plays an important role not only in LPS-induced but also in hemorrhage-induced acute lung injury. This is the first report to implicate GM-CSF in acute hemorrhage-induced lung injury. Although our data seem to support the harmful effect of GM-CSF in systemic inflammation, the positive side of bioactivity of GM-CSF should be considered as well. For example, GM-CSF knockout mice improved bacterial clearance after expression of GM-CSF in the respiratory epithelium (16). In this sense, our data need to be accepted as one of those that emphasize the role of GM-CSF as an augmenting agent of inflammation.
In the in vitro study, co-treatment of GM-CSF with low or medium doses of LPS (1 and 10 ng/mL, respectively) showed no stimulatory effects on the expression of TNF-α and MIP-2 in bone marrow neutrophils. However, the expression of IL-1β, which appears to play an important role in ALI, increased in a dose dependent manner with addition of low or medium (10 ng/mL) doses of GM-CSF. Of note, when GM-CSF was administered with high doses of LPS (100 ng/mL), the expression of three cytokines (TNF-α, MIP-2, or IL-1β) increased; this was dependent on the dose of GM-CSF used (Fig. 5). The incubation time from the administration of GM-CSF and/or LPS to collection of supernatant was only 1 hr. Therefore, GM-CSF might augment the expression of proinflammatory cytokines by its priming effects on neutrophils rather than by delaying apoptosis of neutrophils. In the RT-PCR assay, the combination of GM-CSF with high doses of LPS increased the expression of mRNA of three cytokines (TNF-α, MIP-2, and IL-1β; Fig. 6). These results imply that GM-CSF-induced augmentation of inflammatory cytokine production is regulated at the transcriptional level. In addition, intranuclear translocation of NF-κB was increased in a dose dependent fashion with incremental doses of GM-CSF (Fig. 7). It is well known that NF-κB plays an important role in the mediation of LPS-induced stimulation of proinflammatory cytokines (17). The augmentation of GM-CSF with LPS appears to be mediated by the same mechanism involved in LPS-induced inflammation. The above findings suggest that GM-CSF augments lung inflammation not only by increasing recruitment of neutrophils into lung tissue but also by its priming effects on neutrophils. GM-CSF especially augmented high doses of LPS-induced inflammation, which was consistent, with the in vivo study results.
In summary, GM-CSF knockout mice were more resistant to lung inflammation and lung injury than C57BL control mice, which were induced by LPS-injection or hemorrhage. GM-CSF augmented the inflammatory effect of high dose LPS, which appears to be regulated at the transcriptional level and mediated by NF-κB translocation. In conclusion, GMCSF plays an important role in not only LPS-induced but also hemorrhage-induced ALI by its priming effects on neutrophils.
Figures and Tables
Fig. 1
GM-CSF -/- mice showed decreased infiltration of neutrophils in the lung after LPS injection or hemorrhage. GM-CSF -/- mice and control C57BL mice were treated with low or high doses of LPS (0.5 mg/kg or 5 mg/kg, respectively) injected into the peritoneum. Four hours after the injection of LPS or hemorrhage, mice were sacrificed and lung neutrophil infiltration was evaluated by MPO assay. Each group consisted of five mice.
GM-/-, GM-CSF knockout mice; C57, C57BL control mice; Hmr, hemorrhage; LPS 0.5, LPS 0.5 mg/kg i.p.; LPS 5, LPS 5 mg/kg i.p. *, p<0.05.

Fig. 2
GM-CSF-/- mice were resistant to lung leakage after LPS injection or hemorrhage. GM-CSF -/- mice or control C57BL mice were treated with low or high doses of LPS (0.5 mg/kg and 5 mg/kg, respectively) injected into the peritoneum. Three hours after the injection of LPS or hemorrhage, Evans blue dye (50 mg/kg) was injected into the tail vein. One hour after the injection of dye, mice were sacrificed and the lungs were removed. EBD was extracted from the removed lungs by formamide extraction. Each group consisted of five mice.
GM-/-, GM-CSF knockout mice; C57, C57BL control mice; Hmr, hemorrhage; LPS 0.5, LPS 0.5 mg/kg i.p.; LPS 5, LPS 5 mg/kg i.p. *, p<0.05.

Fig. 3
GM-CSF -/- mice showed decreased levels of TNF-α, MIP-2, and IL-1β in the lung after LPS injection. GM-CSF -/- mice and control C57BL mice were treated with low or high doses of LPS (0.5 mg/kg or 5 mg/kg, respectively) injected into the peritoneum. Four hours after the injection of LPS, mice were sacrificed, and expression of TNF-α, MIP-2 and IL-11β in the lung samples were evaluated by ELISA. Each group consisted of five mice.*, p<0.05.

Fig. 4
GM-CSF -/- mice showed decreased levels of KC, IL-6, and IL-1β in the lung after hemorrhage. Four hours after hemorrhage, mice were sacrificed and the expression of KC, IL-6, and IL-1β in the lung samples was evaluated by ELISA. Each group consisted of five mice. *, p<0.05.

Fig. 5
GM-CSF augments TNF-α, MIP-2, and IL-1β expression in mouse bone marrow neutrophils. Bone marrow neutrophils of control mice (5×105/well) were treated with GM-CSF (0, 1, 10, and 100 ng/mL) in combination with LPS (0, 1, 10, and 100 ng/mL). Four hours after stimulation with GM-CSF and/or LPS, the supernatant was collected and the expression of TNF-α, MIP-2, and IL-1β was evaluated by ELISA. This experiment was done three times independently and the displayed data are mean values. *, p<0.05

Fig. 6
GM-CSF augments LPS-induced mRNA expression of TNF-α, MIP-2, and IL-1β in mouse bone marrow neutrophils. LPS (100 ng/mL) and incremental concentration of GM-CSF (0, 1, 10, and 100 ng/mL) was added to bone marrow neutrophils of control mice (1×106/microtube). One hour later, the microtube was centrifuged for 12,000 g at 4℃ and total RNA was collected. The mRNA expression of TNF-α, MIP-2, and IL-1β was measured by RT-PCR. This experiment was done three times independently and the displayed data are mean values.
LPS 100, LPS 100 ng/mL; LPS 100/GM1, LPS 100 ng/mL+GM-CSF 1 ng/mL; LPS 100/GM10, LPS 100 ng/mL+GM-CSF 10 ng/mL; LPS 100/GM100, LPS 100 ng/mL+GM-CSF 100 ng/mL. *, p<0.05.

Fig. 7
GM-CSF augments LPS-induced nuclear translocation of NF-κB in mouse bone marrow neutrophils. Isolated neutrophils were allocated into 1.5 mL microtubes (107 cells/tube). LPS (100 ng/mL) and incremental concentrations of GM-CSF (0, 1, 10, and 100 ng/mL) were added to bone marrow neutrophils of control mice. One hour later, the microtube was centrifuged at 12,000 g and 4℃ and the nuclear protein was extracted for EMSA. The upper figure and lower graph are representatives of three independent experiments. GM, GM-CSF.
References
1. Fibbe WC, Daha MR, Hiemstra PS, Duinkerken N, Lurvink E, Ralph P, Altrock BW, Kaushansky K, Willemze R, Falkenburg JH. Interleukin1 and poly(rl)poly(rC) induce production of granulocyt CSF, macrophage CSF and granulocyte-macrophage CSF by human endothelial cells. Exp Hematol. 1989. 17:229–234.
2. Strieter RM, Kunkel SL, Showell HJ, Remick DG, Phan SH, Ward PA, Marks RM. Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science. 1989. 243:1467–1469.

3. Gasson JC, Weisbart RH, Kaufman SE, Clark SC, Hewick RM, Wong GG, Golde DW. Purified human granulocyte-macrophage colony-stimulating factor: direct effects on neutrophils. Science. 1984. 226:1339–1342.
4. Weisbart RH, Golde DW, Clark SC, Wong GG, Gasson JC. Human granulocyte-macrophage colony-stimulating factor is a neutrophil activator. Nature. 1985. 314:361–363.

5. Arnberg H, Letocha H, Nou F, Westlin JF, Nilsson S. GM-CSF in chemotherapy-induced febrile neutropenia--a double-blind randomized study. Anticancer Res. 1998. 18:1255–1260.
6. Lopez AF, Williamson DJ, Gamble JR, Begley CG, Harlan JM, Klebanoff SJ, Waltersdorph A, Wong G, Clark SC, Vadas MA. Recombinant human granulocyte-macrophage colony-stimulating factor stimulates in vitro mature human neutrophil and eosinophil function, surface receptor expression, and survival. J Clin Invest. 1986. 78:1220–1228.

7. Verhoef G, Boogaerts M. Treatment with granulocyte-macrophage colony-stimulating factor and the adult respiratory distress syndrome. Am J Hematol. 1991. 36:285–287.
8. Tiegs G, Barsig J, Matiba B, Uhlig S, Wendel A. Potentiation by granulocyte macrophage colony-stimulating factor of lipopolysaccharide toxicity in mice. J Clin Invest. 1994. 93:2616–2622.

9. Sauaia A, Moore FA, Moore EE, Lezotte DC. Early risk factors for postinjury multiple organ failure. World J Surg. 1996. 20:392–400.

10. Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT, Dickersin GR, Bachurski CJ, Mark EL, Whitsett JA. Involvement of granulocyte-macrophage colony-stimulating factor in pulmonary homeostasis. Science. 1994. 264:713–716.

11. Kim JY, Park JS, Strassheim S, Douglas I, del Valle FD, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, Ishizaka A, Abraham E. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol. 2005. 288:L958–L965.

12. Pelaez A, Bechara RI, Joshi PC, Brown LA, Guidot DM. Granulocyte/macrophage colony-stimulating factor treatment improves alveolar epithelial barrier function in alcoholic rat lung. Am J Physiol Lung Cell Mol Physiol. 2004. 286:L106–L111.

13. Orozco H, Arch J, Medina-Franco H, Pantoja JP, Gonzalez QH, Vilatoba M, Hinojosa C, Vargas-Vorackova F, Sifuentes-Osornio J. Molgramostim (GM-CSF) associated with antibiotic treatment in nontraumatic abdominal sepsis: a randomized, double-blind, placebo-controlled clinical trial. Arch Surg. 2006. 141:150–153.
14. Basu S, Dunn AR, Marino MW, Savoia H, Hodgson G, Lieschke GJ, Cebon J. Increased tolerance to endotoxin by granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1997. 159:1412–1417.
15. Kamiya K, Kono T, Iwamoto J, Yoneda M, Kotani H, Kasai S. The cytoprotective role of lipopolysaccharide-induced nitric oxide against liver damage during early phase of endotoxemia in rats. Shock. 2000. 14:229–233.





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download