Abstract
Fabrazyme has been widely used for treatment of Fabry disease since its approval by the U.S. Food and Drug Administration in 2003. This study was undertaken to assess the short-term efficacy and safety of enzyme replacement therapy (ERT) for Fabry disease in Korea. Eight male patients and three female symptomatic carriers aged 13 to 48 yr were included. Fabrazyme was administered by intravenous infusion at a dose of 1 mg/kg every 2 weeks. Plasma and urine globotriaosylceramide (GL-3) levels, serum creatinine, creatinine clearance, and 24-hr urine protein levels were measured every 3 months. Kidney biopsies, ophthalmologic exams, and pure tone audiometry were performed before and 1 yr after ERT. Kidney function, including serum creatinine, creatinine clearance, and the 24-hr urine protein level, remained stable during ERT. Plasma and urine GL-3 levels were reduced within 3 to 6 months of ERT initiation. Microvascular endothelial deposits of GL-3 were decreased from renal biopsy specimens after 1 yr of treatment. The severity of sensorineural hearing loss and tinnitus did not improve after ERT. ERT is safe and effective in stabilizing renal function and clearing microvascular endothelial GL-3 from kidney biopsy specimen in Korean patients with Fabry disease.
Fabry disease (OMIM #301500) is an X-linked lysosomal storage disorder caused by a deficiency of α-galactosidase A (EC 3.2.1.22) (1). Progressive systemic deposition of globotriaosylceramide (GL-3) in podocytes causes proteinuria. Deposition in cardiomyocytes causes cardiac hypertrophy, and deposition in vascular endothelial cells, in pericytes, and in smooth muscle cells of the vascular system leads to ischemia and infarction. GL-3 accumulates in lysosomes of the vascular endothelial, smooth muscle cells, epithelial, perithelial, reticuloendothelial, myocardial, ganglion, and perineural cells (2). Deposition of GL-3 occurs throughout the nephrons and renal vasculature, leading to progressive glomerular injury associated with mesangial widening and segmental and global glomerulosclerosis (3). Early symptoms of Fabry disease include acroparesthesias, angiokeratoma, and corneal opacities (4). In elderly patients, accumulation of glycolipid progresses to chronic renal failure and stroke. Death in the fifth decade was the usual common outcome in affected males before the advent of dialysis and transplantation (5). Female heterozygous carriers are usually asymptomatic, but can be affected with a wide spectrum of clinical abnormalities because of random X-chromosomal inactivation, generally at a later age than affected males (6). Atypical variants of hemizygous Fabry disease were found among patients who manifested with unexplained left ventricular hypertrophy (7). Most non-classical variants with attenuated disease do not have endothelial glycosphingolipid deposition, do not develop renal failure and, after living a normal lifespan, might die due to late cardiac manifestations of the disease. Following Gaucher disease, Fabry disease is the second most common lysosomal storage disorder with an estimated frequency of 1 in 117,000 live births. The prevalence in males is estimated as 1:40,000 to 60,000 (2, 8). The register contains data on 8 classical male patients and 3 female symptomatic carriers in this study. However, the precise prevalence of Fabry disease is unknown in Korea. It is quite possible that the number of atypically symptomatic patients with renal or cardiac variants of Fabry disease might be greater than current estimates (9).
After approval of Fabrazyme® (Genzyme Corp., Cambridge, MA, U.S.A.) by the European Agency for the Evaluation of Medicinal Products in 2001 and the U.S. Food and Drug Administration in 2003, it has been widely used for treatment of Fabry disease. Phase 1 and 2 trials with recombinant α-galactosidase A have demonstrated enzyme replacement therapy (ERT) is safe and effective in clearing the plasma and endothelial deposits of GL-3 from target tissues (10). Several clinical trials have shown that ERT in Fabry disease is efficacious in decreasing pain, stabilizing renal function, and clearing glycolipids stored in the lysosomes (11, 12). This study was undertaken to investigate the short-term clinical efficacy of ERT for 8 classical male patients and 3 symptomatic female carriers with Fabry disease in Korea.
To date, 11 patients (8 classical male patients and 3 symptomatic female carriers) have been under ERT after obtaining informed consent. The patients were between 13 and 48 yr of age (mean 28.6±11.89 yr). All patients were examined for changes in angiokeratoma and other changes in clinical symptoms at each visit. Patients' ophthalmologic status was evaluated with a complete assessment that included a slit-lamp examination every 6 months by an ophthalmologist. Electrocardiogram and pure tone audiometry were also performed before ERT and every 6 months during treatment. Renal function was evaluated by measuring serum creatinine, creatinine clearance, and 24-hr urine protein levels every 3 months. No patients received any concomitant medication, such as antihypertensive drugs or lipid-lowering drugs during the study.
The plasma α-galactosidase A activity was measured by fluorometric assay using 10 mM 4-methyumbelliferyl-α-Dgalactoside (Sigam-Aldrich, St. Louis, MO, U.S.A.) as a substrate. Leukocytes were isolated from peripheral blood collected in an EDTA tube. After sonication with Virsonic100 (Virtis, Gardiner, NY, U.S.A.), the leukocyte supernatant with 0.1 mL of the substrate was incubated at 37℃ for 30 min. For preclusion of α-galactosidase B (GALB) activity, 0.1 M N-acetylgalactosamine was added to the reaction mixture as an inhibitor of GALB. Reactions were stopped by adding 1.3 mL of 0.17 M glycine-carbonate buffer at pH 9.8. The fluorescence at an excitation wavelength of 360 nm and an emission wavelength of 415 nm was read. Normal ranges in our laboratory were from 45 to 85 nmoles/hr/mg in leukocytes (13).
Plasma and urine were prepared using a modification of the Bligh-Dyer method (14). Plasma was mixed with distilled water at a ratio of 1:4. A 5-µL working internal standard solution of 2.5 µg/mL C17:0 GL-3 was added, then 80% dioxane was mixed into the solution, which was then vortex-mixed for 30 sec and centrifuged at 12,000 rpm for 5 min. The upper layer was transferred to an injection vial for tandem mass spectrometry (MS/MS) analysis. Urine was prepared as plasma in a 1:50 dioxane dilution ratio. A diluted sample was used directly for acquisition of positive-ion electrospray ionization (ESI) mass spectra. MS/MS after ESI was performed as the previously described method after fast atom bombardment (FAB) ionization (15). The 8 GL-3 isomers and C17:0 GL-3 were separated on a C8 guard cartridge column (4×3 mm internal diameter; phenomenex) prior to quantification by ESI-MS/MS. Normal ranges for plasma GL-3 were from 3.88 to 9.87 µg/mL and for urine GL-3 were from 0.008 to 0.898 µg/mg Cr. During ERT, GL-3 levels were measured in plasma and urine every 3 months.
Genomic DNA was isolated from peripheral blood leukocytes using a Puregene DNA isolation kit (Gentra, Minneapolis, MN, U.S.A.). Seven exons of GLA and their intronic flanking sequences were amplified by polymerase chain reaction (PCR) with seven sets of previously described primers, followed by single-strand conformational polymorphism analysis and direct sequencing (16). DNA sequencing was carried out using the same primers used in PCR with a BigDye Terminator V3.0 Cycle Sequencing Ready reaction kit (Applied Biosystems, Foster city, CA, U.S.A.). Electrophoresis and analysis of the reaction mixtures were performed on an ABI 3100 Genetic analyzer (Applied Biosystems).
Histologic and ultrastructural studies using standard procedures were employed to evaluate pathologic changes and the degree of GL-3 deposition before and 1 yr after the first onset of ERT. Kidney specimens were obtained in 3 out of 11 patients by ultrasound-guided needle biopsy. Kidney biopsies were not performed in the other patients due to their short follow-up periods less than one year. The specimens for histological examination were fixed in Bouin fixative, embedded in paraffin, and cut into 2 µm sections. Subsequently, hematoxylin and eosin, periodic acid-Schiff, periodic acid-silver methenamine, Luxol-fast blue, and Masson's trichrome staining were performed. For electron microscopy, 2.5% gluteraldehyde-fixed tissues were post-treated with osmium tetraoxide, embedded in Epon, cut at 50-80 nm, double-stained with uranyl acetate and lead citrate, and examined under a JEOL 1200EX-II transmission electron microscope. For the preparation of semi-thin sections, tissues were cut at 1 µm and stained with toluidine blue.
On ultrastructural studies, the vascular endothelial deposits of GL-3 was scored from 0 to 3 as previously described (11): 0; no inclusions, trace, one small granule, 1; multiple discrete granules, 2; single or multiple aggregates of granules, 3; aggregates of granules causing distortion of luminal endothelial surface. Renal histology was examined by the same pathologist in all three patients. Pathologist was blinded to patient identities and the time points at which the specimens had been obtained.
Agalsidase beta (Fabrazyme®; Genzyme Corp., Cambridge, MA, U.S.A.) was intravenously infused at a dose of 1 mg/kg every 2 weeks. The enzyme was diluted in a 0.07 mg/mL of concentration with normal saline and was administered at a rate of 15 mg/hr. The duration of treatment ranged from 4 to 27 months.
Statistical analyses were performed using SPSS version 12.0 for Windows. Changes before and after treatment were analyzed by a Wilcoxon signed rank tests. Differences in parameters at more than two treatment time points were analyzed for significance using repeated measures ANOVA. p values less than 0.05 were considered to be statistically significant.
The age at initial ERT ranged from 13 to 48 yr. Five out of 11 Fabry patients exhibited neurologic manifestations including tinnitus, dizziness, and sensorineural hearing loss. The most common presenting symptoms and signs were acroparesthesia, hypohidrosis, and proteinuria. Patients 1, 6, and 7 have received diphenylhydantoin and carbamazepine for pain relief. Left ventricular hypertrophy was observed before ERT in 6 patients on electrocardiogram or echocardiogram. Corneal clouding was observed in 9 patients on slit lamp examination. All female carriers experienced acroparesthesias in childhood. They exhibited mild clinical manifestations such as neuronopathic pain, hypohidrosis, corneal clouding, and lymphedema of legs. Two patients (patients 6 and 8) were diagnosed at pediatric ages (13-17 yr of age) (Table 2). ERT has been initiated as soon as they were diagnosed. Two pediatric patients manifested with acroparesthesia, anhidrosis, left ventricular hypertrophy, corneal clouding, and gastrointestinal symptoms such as vomiting and diarrhea. After one year of treatment, high-frequency sensorineural hearing loss and tinnitus were not ameliorated in all affected patients. There was no remarkable improvement in angiokeratoma of the skin, corneal clouding, and left ventricular hypertrophy.
Serum creatinine levels were normal, except for patient 5, and remained stable throughout the treatment periods (Fig. 1A). Patient 5 was diagnosed with Fabry disease after kidney transplantation, and his serum creatinine levels also remained stable. Sequential changes in creatinine clearance revealed some inter- and intra-individual variations during ERT. However, these changes were statistically not significant (p>0.05) (Fig. 1B). Five patients had increased protein excretion of more than 150 mg/day before treatment. There were no significant changes in the degree of proteinuria during ERT (p>0.05) (Fig. 1C).
All patients exhibited increased plasma GL-3 levels, except for two female heterozygotes. The GL-3 levels were reduced to the normal range within 3 to 6 months of ERT onset, with the exception of two patients (patients 4 and 11) (Fig. 2A). Urine GL-3 levels were also elevated, but reduced significantly within 6 to 9 months after ERT onset except patient 4 (Fig. 2B).
Pre- and post-treatment kidney biopsy findings were compared in three patients (patients 1, 6, and 7). The histologic features evaluated were summarized in Table 3. On pathologic examination, all patients revealed cytoplasmic foamy vacuolization and GL-3 deposition of podocytes, tubular epithelial cells, endothelial cells, and vascular smooth muscle cells, which are the characteristic pathologic features of Fabry disease. In patients 1 and 6, the GL-3 deposition was markedly decreased in the vascular endothelial cells after ERT, although the glomerular and tubulointerstitial features were not changed (Fig. 3). However, in patient 7, the GL-3 deposition in the vascular endothelial cells was not decreased and the glomerular and tubulointerstitial changes were advanced.
Two patients (patients 3 and 7) experienced mild fever and chest tightness as infusion-association reactions on their first infusion. They have been on a regular pre-infusion medication including acetaminophen and hydroxyzine to prevent infusion reactions and these symptoms were relieved by pre-infusion medication.
This is the first report of ERT in Korean patients with Fabry disease. The wide spectrum of clinical and laboratory findings documented in this study were similar to previous reports in most of the males and females (17, 18). Although the period of treatment and the age of the subjects vary considerably, ERT is safe and effective in stabilizing renal functions, decreasing GL-3 levels in plasma and urine, and clearing GL-3 deposits from kidney biopsy specimens without significant adverse events in patients with Fabry disease.
Fabry disease is diagnosed by demonstration of deficient α-galactosidase A activity in plasma or leukocytes and mutation analysis of the GLA gene. Patients with residual enzyme activity have a milder, variant phenotype (19). In female heterozygotes, a very low α-galactosidase A level is also diagnostic of the carrier state. However, normal or near-normal enzyme activity does not rule out the possibility that a female is a carrier because of random X-chromosomal inactivation. Thus, all girls and women at risk for carrying the disease gene should be determined their status by molecular studies (20).
The α-galactosidase A is encoded by the GLA gene that contains 7 exons located on Xq22.1 (21). Over 350 mutations have been identified in the Human Gene Mutation Database (http://www.hgmd.org/). Six different mutations of the GLA gene have been identified in 6 families of Fabry disease (Table 1). Two mutations (p.L268fsX1 and p.D266N) were previously reported as novel by the authors (22). The mutations in three patients (patients 1, 5, and 11) were de novo without mutations of the GLA gene in other family members. Genetic counseling should be provided to inform other family members of the availability of diagnostic testing and early treatment. The periods from the onset of symptoms to the ages of diagnosis ranged from 1 to 33 yr.
The optimal goal of ERT is to preserve normal renal function by early clearance of GL-3 endothelial deposits and prevention of further deposits (23). Clinical trials have demonstrated the safety and effectiveness of ERT (11). ERT resulted in decreased plasma and urine GL-3 levels even after a short-term treatment, and reduced GL-3 accumulation in various organs and tissues (24). Elevations and changes in urine glycolipids were less pronounced in heterozygotes and in recipients of a renal allograft (25). It admits no doubt that ERT should be initiated in carriers with substantial disease manifestations as well as all affected male patients with Fabry disease as early as possible to prevent irreversible major organ damage (12, 26-28). ERT was safe and effective in clearing of GL-3 and improvement of autonomic function in children with Fabry disease (29). Urinary excretion of GL-3 has not been reduced except in patient 4, although his renal function has remained stable during ERT. Long-term follow-up is needed for the evaluation of effects on urinary GL-3 excretion in this patient.
Clinical symptoms, such as acroparesthesia, pain crisis, hypohidrosis, and diarrhea, have been reported to be improved by ERT (30). Improvement of pain and anhidrosis was not assessed in this report due to its subjective nature and relatively short-term follow-up periods to evaluate the effectiveness to neurologic symptoms. Although pain scores were not assessed, pain crisis in patient 1 decreased during ERT. Angiokeratoma did not change after 24 months of ERT (30). In this report, corneal clouding, angiokeratoma, acroparesthesia, and sensorineural hearing loss did not change during ERT. Although there are a few reports on the effects of ERT on sensorineural hearing loss, it appears to be reversed after long-term ERT (31, 32). Pure tone audiometry should be followed-up in these patients after long-term treatment. The major limitation of the use of ERT for Fabry disease is the inability of large molecules such as enzymes to cross the blood-brain barrier (33). Therefore, it is controversial as to whether the use of ERT could improve central nervous system manifestations. Six patients with left ventricular hypertrophy did not reveal any change their electrocardiogram or echocardiogram findings during short-term ERT. Cardiac contractility and left ventricular mass improved after 18 months of ERT in kidney transplant patients (34).
There were no significant changes in renal function during short-term treatment periods in this study. The clinical efficacy of ERT was associated with stabilization of renal function, a significant reduction in the GL-3 level, and improvement in renal pathology (34). Therefore, ERT is recommended in patients who received renal transplantation to improve quality of life and to prevent cardiac and cerebrovascular complications (26, 30). Patient 5 has been treated with ERT after renal transplantation without significant adverse effects or any complications of Fabry disease. Previous studies have also demonstrated that ERT stabilized renal function after 30 months of an open-label extension trial study (12). Renal pathologic findings in this study also revealed persistent GL-3 deposits in podocytes. Despite decreased plasma and urine GL-3 levels, there were no significant changes in urinary protein excretion and serum creatinine levels.
The occurrence of immunoglobulin G antibodies results in hypersensitivity reactions in some patients, necessitating reduction in the rate of infusion time and/or the use of premedications such as antihistamines or corticosteroids (11, 34). Although the vast majority of patients on agalsidase beta treatment develop antibodies, antibodies against agalsidase were not measured in this study. Whether the inhibition of recombinant human α-galactosidase A activity by IgG antibodies has a significant clinical effect or not is unclear (35). Infusion-associated reactions have been observed in two patients, which were easily treated with acetaminophen and hydroxyzine.
In summary, this study demonstrates that the clinical effects of ERT were associated with a significant reduction in the serum and urine GL-3 levels, and clearance of the accumulated GL-3 from the vascular endothelium of biopsied kidney. It is anticipated that ERT will stabilize the disease and preserve kidney function, thereby preventing the cardiac and cerebrovascular complications in patients with Fabry disease. Therefore, ERT should be initiated as early as possible in affected males and symptomatic carrier females before irreversible damage has occurred.
Figures and Tables
 | Fig. 1Renal function of patients during enzyme replacement therapy. Serum creatinine (A), creatinine clearance (B), and 24-hr urine protein levels (C) remained stable without aggravation or improvement (p>0.05). Data are presented as mean±SD. |
 | Fig. 2Sequential changes in the GL-3 concentration in plasma (A) and urine (B) during enzyme replacement therapy. |
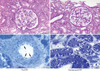 | Fig. 3Pathologic features before (A, C) and after (B, D) ERT. No significant changes were identified the glomerulus and tubulointerstitium on histologic examination. However, the vascular endothelial GL-3 depositions (arrows) were markedly decreased after the ERT (from patient 1; A and B, PAS staining at ×400 magnification; C and D, toluidine blue staining of semithin sections at ×1,000 magnification). |
References
2. Desnick RJ, Ioannou YA, Eng CM. Scriver CR, Beaudet AL, Sly WS, editors. α-galactosidase A deficiency: Fabry disease. The Metabolic and Molecular Bases of Inherited Disease. 2001. 8th edn. New York: McGraw-Hill;3733–3774.
3. Sheth KJ, Roth DA, Adams MB. Early renal failure in Fabry's disease. Am J Kidney Dis. 1983. 2:651–654.

4. Peters FP, Sommer A, Vermeulen A, Cheriex EC, Kho TL. Fabry's disease: a multidisciplinary disorder. Postgrad Med J. 1997. 73:710–712.

5. Branton MH, Schiffmann R, Sabnis SG, Murray GJ, Quirk JM, Altarescu G, Goldfarb L, Brady RO, Balow JE, Austin Iii HA, Kopp JB. Natural history of Fabry renal disease: influence of alpha-galactosidase A activity and genetic mutations on clinical course. Medicine (Baltimore). 2002. 81:122–138.
6. Gupta S, Ries M, Kotsopoulos S, Schiffmann R. The relationship of vascular glycolipid storage to clinical manifestations of Fabry disease: a cross-sectional study of a large cohort of clinically affected heterozygous women. Medicine (Baltimore). 2005. 84:261–268.
7. Nakao S, Takenaka T, Maeda M, Kodama C, Tanaka A, Tahara M, Yoshida A, Kuriyama M, Hayashibe H, Sakuraba H, Tanaka H. An atypical variant of Fabry's disease in men with left ventricular hypertrophy. N Engl J Med. 1995. 333:288–293.

8. Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999. 281:249–254.

9. Thadhani R, Wolf M, West ML, Tonelli M, Ruthazer R, Pastores GM, Obrador GT. Patients with Fabry disease on dialysis in the United States. Kidney Int. 2002. 61:249–255.

10. Eng CM, Banikazemi M, Gordon RE, Goldman M, Phelps R, Kim L, Gass A, Winston J, Dikman S, Fallon JT, Brodie S, Stacy CB, Mehta D, Parsons R, Norton K, O'Callaghan M, Desnick RJ. A phase 1/2 clinical trial of enzyme replacement in Fabry disease: pharmacokinetic, substrate clearance, and safety studies. Am J Hum Genet. 2001. 68:711–722.

11. Eng CM, Guffon N, Wilcox WR, Germain DP, Lee P, Waldek S, Caplan L, Linthorst GE, Desnick RJ; International Collaborative Fabry Disease Study Group. Safety and efficacy of recombinant human α-galactosidase A replacement therapy in Fabry's disease. N Engl J Med. 2001. 345:9–16.

12. Wilcox WR, Banikazemi M, Guffon N, Waldek S, Lee P, Linthorst GE, Desnick RJ, Germain DP; International Fabry Disease Study Group. Long-term safety and efficacy of enzyme replacement therapy for Fabry disease. Am J Hum Genet. 2004. 75:65–74.
13. Schiffmann R, Murray GJ, Treco D, Daniel P, Sellos-Moura M, Myers M, Quirk JM, Zirzow GC, Borowski M, Loveday K, Anderson T, Gillespie F, Oliver KL, Jeffries NO, Doo E, Liang TJ, Kreps C, Gunter K, Frei K, Crutchfield K, Selden RF, Brady RO. Infusion of alpha-galactosidase A reduces tissue globotriaosylceramide storage in patients with Fabry disease. Proc Natl Acad Sci USA. 2000. 97:365–370.
14. Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959. 37:911–917.

15. Kayganich KA, Murphy RC. Fast atom bombardment tandem mass spectrometric identification of diacyl, alkylacyl, and alk-1-enylacyl molecular species of glycerophosphoethanolamine in human polymorphonuclear leukocytes. Anal Chem. 1992. 64:2965–2971.

16. Blanch LC, Meaney C, Morris CP. A sensitive mutation screening strategy for Fabry disease: detection of nine mutations in the alpha-galactosidase A gene. Hum Mutat. 1996. 8:38–43.
17. MacDermot KD, Holmes A, Miners AH. Anderson-Fabry disease: clinical manifestations and impact of disease in a cohort of 98 hemizygous males. J Med Genet. 2001. 38:750–760.

18. Whybra C, Kampmann C, Willers I, Davies J, Winchester B, Kriegsmann J, Bruhl K, Gal A, Bunge S, Beck M. Anderson-Fabry disease: clinical manifestations of disease in female heterozygotes. J Inherit Metab Dis. 2001. 24:715–724.

19. Yoshitama T, Nakao S, Takenaka T, Teraguchi H, Sasaki T, Kodama C, Tanaka A, Kisanuki A, Tei C. Molecular genetic, biochemical, and clinical studies in three families with cardiac Fabry's disease. Am J Cardiol. 2001. 87:71–75.

21. Kornreich R, Desnick RJ, Bishop DF. Nucleotide sequence of the human alpha-galactosidase A gene. Nucleic Acids Res. 1989. 17:3301–3302.
22. Lee JK, Kim GH, Kim JS, Kim KK, Lee MC, Yoo HW. Identification of four novel mutations in five unrelated Korean families with Fabry disease. Clin Genet. 2000. 58:228–233.

23. Schiffmann R, Kopp JB, Austin HA 3rd, Sabnis S, Moore DF, Weibel T, Balow JE, Brady RO. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA. 2001. 285:2743–2749.
24. Schiffmann R, Floeter MK, Dambrosia JM, Gupta S, Moore DF, Sharabi Y, Khurana RK, Brady RO. Enzyme replacement therapy improves peripheral nerve and sweat function in Fabry disease. Muscle Nerve. 2003. 28:703–710.

25. Whitfield PD, Calvin J, Hogg S, O'Driscoll E, Halsall D, Burling K, Maguire G, Wright N, Cox TM, Meikle PJ, Deegan PB. Monitoring enzyme replacement therapy in Fabry disease-role of urine globotriaosylceramide. J Inherit Metab Dis. 2005. 28:21–33.

26. Desnick RJ, Brady R, Barranger J, Collins AJ, Germain DP, Goldman M, Grabowski G, Packman S, Wilcox WR. Fabry disease, an under-recognized multisystemic disorder: expert recommendations for diagnosis, management, and enzyme replacement therapy. Ann Intern Med. 2003. 138:338–346.

27. Ries M, Gupta S, Moore DF, Sachdev V, Quirk JM, Murray GJ, Rosing DR, Robinson C, Schaefer E, Gal A, Dambrosia JM, Garman SC, Brady RO, Schiffmann R. Pediatric Fabry disease. Pediatrics. 2005. 115:e344–e355.

28. Ries M, Ramaswami U, Parini R, Lindblad B, Whybra C, Willers I, Gal A, Beck M. The early clinical phenotype of Fabry disease: a study on 35 European children and adolescents. Eur J Pediatr. 2003. 162:767–772.

29. Ries M, Clarke JT, Whybra C, Timmons M, Robinson C, Schlaggar BL, Pastores G, Lien YH, Kampmann C, Brady RO, Beck M, Schiffmann R. Enzyme-replacement therapy with agalsidase alfa in children with Fabry disease. Pediatrics. 2006. 118:924–932.

30. Pisani A, Spinelli L, Sabbatini M, Andreucci MV, Procaccini D, Abbaterusso C, Pasquali S, Savoldi S, Comotti C, Cianciaruso B. Enzyme replacement therapy in Fabry disease patients undergoing dialysis: effects on quality of life and organ involvement. Am J Kidney Dis. 2005. 46:120–127.

31. Hajioff D, Enever Y, Quiney R, Zuckerman J, Mackermot K, Mehta A. Hearing loss in Fabry disease: the effect of agalsidase alfa replacement therapy. J Inherit Metab Dis. 2003. 26:787–794.

32. Hajioff D, Hegemannn S, Conti G, Beck M, Sunder-Plassmann G, Widmer U, Mehta A, Keilmann A. Agalsidase alpha and hearing in Fabry disease: data from the Fabry Outcome Survey. Eur J Clin Invest. 2006. 36:663–667.

33. Wilcox WR. Lysosomal storage disorders: the need for better pediatric recognition and comprehensive care. J Pediatr. 2004. 144:5 Suppl. S3–S14.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download