Abstract
A number of case reports on occupational asthma caused by herbal medicines have been issued, for example, on Sanyak, Chunkung, Banha, and Brazilian ginseng. Recently, cases of occupational asthma induced by Sanyak and Korean ginseng have been reported, but the pathogenic mechanisms involved are unknown. This study was carried out to evaluate the immunologic mechanism underlying Korean ginseng-induced occupational asthma. A patient engaged in Korean ginseng wholesale was referred for recurrent dyspnea, wheezing, and nasal symptoms, which were aggravated at work. Allergen bronchial provocation testing to Korean ginseng extract showed a typical immediate response, and skin prick testing to Korean ginseng extract also showed a strong positive response. Moreover, serum-specific IgE levels to Korean ginseng extract were significantly higher than in controls. Enzyme-linked immunosorbent assay (ELISA) inhibition tests showed a dose-dependent inhibition by Korean ginseng, but not by Dermatophagoides farinae, wheat flour, or Chinese balloon flower. Sodium dodecylsulfate-poly-acrylamide gel electrophoresis (SDS-PAGE) and immunoblotting revealed four specific Immunoglobulin E (IgE) binding components at 26, 30, 47, and 60 kDa, which were not bound by control sera. These results strongly suggest that occupation asthma induced by Korean ginseng is induced via an IgE-mediated mechanism.
Occupational asthma is induced by many agents in workplaces. A number of herbal medicines, e.g., Sanyak (1), Chunkung (2), Banha (3), and Brazilian ginseng (4) have been reported to be causative agents of occupational asthma. Recently, one case report of Sanyak and Korean ginseng-induced occupational asthma was reported (confirmed by bronchial provocation testing) (5). Although skin prick testing with Korean ginseng extract was weakly positive in this reported case, enzyme-linked immunosorbent assay (ELISA) result for serum anti-Korean ginseng-specific immunoglobulin (IgE) and no IgE binding component was detected by immunoblotting (5). Here, we report a case of Korean ginseng-induced occupational asthma with a strong positive skin prick test to ginseng extract and an elevated serum anti-Korean ginseng-specific IgE level. The IgE binding components of Korean ginseng were also identified in this case. To the best of our knowledge, this is the first report to reveal the pathogenic mechanism of Korean ginseng-induced occupational asthma.
In May 2005, a 34-yr-old woman was referred for recurrent dyspnea and nasal symptoms. The patient had allergic rhinitis for 9 yr. She presented with abdominal pain and diarrhea after eating many different foods (e.g., tomatoes, bananas, oranges, and oysters) and experienced generalized urticaria and angioedema after eating persimmon. Five years previously, she had started working at a Korean ginseng wholesale premise, where she was exposed to dried ginseng and ginseng dust. After starting the work, she developed frequent rhinorrhea, sneezing, and nasal obstruction. Six months previously, she had developed dyspnea and wheezing on a daily basis, and these were aggravated at work, but improved after a weekend break. She had no allergic symptoms after ingesting non-dried ginseng or steamed red ginseng. During her first visit, she had no symptoms, presumably because she had not been working for a week. Her physical examination was normal, and according to laboratory tests, her peripheral eosinophil count was 572/µL and total IgE 1,050 U/mL. Forced expiratory volume in 1 sec (FEV1) was 3,010 mL (106%) and forced vital capacity (FVC) 3,150 mL (96%), with an FEV1/FVC ratio of 95.5%. Skin prick testing results were as follows: Dermophagoides farinae (6×7 mm), cat (7×14 mm), wheat flour (3.5×5 mm), barley (4×4 mm), Chinese balloon flower (7×14 mm), wheat bran (5×5 mm), alder (3×5 mm), beech (2×3 mm), oak (2×3 mm), orchard (2×3 mm), and positive control-histamine (4×4.5 mm).
Airway hyperresponsiveness was confirmed by bronchial provocation testing to methacholine (PC20=14.77 mg/mL).
Ginseng was obtained from the patient's employer and was prepared as previously described (6). In brief, ginseng was cut into small pieces and extracted into phosphate-buffered saline (PBS; pH 7.5) 1:5 wt/vol at 4℃ for 24 hr. The extract was then centrifuged at 15,000 rpm at 4℃ for 30 min. The supernatant obtained was dialyzed (at a molecular weight cut-off of 6 kDa) against 4 L of normal saline at 4℃ for 48 hr, and this was then used as a crude extract. Extract protein concentrations were determined using the bicinchoninic acid (BCA) assay, according to the manufacturer's instructions (Pierce, Rockford, IL, U.S.A.). Extract was used for skin prick tests and specific bronchial challenge testing.
Normal saline (1 mL) was administered using a DeVil-biss 646 nebulizer (CS & M Instrument Co., Doylestown, PA, U.S.A.) connected to a dosimeter. The subject was asked to breathe the aerosol with tidal breathing, and this was followed by ginseng extract. FEV1 and FVC were measured using a spirometer (Multispiro-SX, Irvine, CA, U.S.A.) before and 10 min after each inhalation, every 10 min during the first hour, and then hourly for the next 8 hr after challenge.
The presence of specific IgE antibody to ginseng extract was determined by ELISA, as previously described (6). In brief, microtiter plates (NUNC; immunoplates, Roskilde, Denmark) were coated with 50 µL of Korean ginseng extract (100 µg/mL), and then incubated with 50 µL of either the patient's serum or undiluted sera from five asthmatics with negative skin prick test responses to common aeroallergens and to ginseng extract. After washing, the immunoplate was incubated with 1:1,000 vol/vol biotin-labeled goat anti-human IgE antibody (Sigma, St. Louis, MO, U.S.A.) and then with 1: 1,000 vol/vol streptavidin-peroxidase (Sigma). After washing, 75 µL of TMB solution (one tablet of 3,3,5,5-tetramethylbenzidine in 10 mL of phosphate citrate buffer containing 2 µL of 30% hydrogen peroxide) was added as substrate, and 75 µL of 2.5 NH2SO4 was added to stop the reaction 5 min later. The calorimetric reaction was quantified by measuring absorbance at 450 nm using an ELISA reader. All assays were performed in triplicate.
Competitive ELISA inhibition tests were performed to determine the specificity of IgE binding to ginseng antigen. In brief, 50 µL of patient serum was preincubated with ginseng extract, Dermatophagoides farinae, wheat flour, or Chinese balloon flower extracts for 1 hr at room temperature. The mixtures were then incubated on a ginseng-coated microtiter plate for 2 hr. The same steps were then followed as for ELISA. After studying control samples, where equal volumes of PBS were preincubated instead of inhibitors, the inhibition of the specific IgE binding was expressed as: 100- (absorbance of samples preincubated with allergens/absorbance of samples preincubated with PBS) ×100 (%).
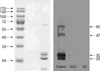
The protein composition pattern and specific IgE binding components of ginseng were analyzed by SDS-PAGE and by immunoblotting using the patient's serum. In brief, 25 µg of ginseng extract was loaded and separated by 12% SDS-PAGE. After electrophoresis, the gel was stained with Coomassie Brilliant Blue R-250 solution (Bio-Rad, Hercules, CA, U.S.A.) and analyzed. To identify the specific IgE binding components of ginseng extracts, immunoblot analysis was performed using the patient's serum. After separating the proteins by SDS-PAGE, they were electrophoretically transferred from the gel to a nitrocellulose membrane using a Bio-Rad Trans-Blot system. Blocking was done by incubation the membrane in a solution of 10% non-fat dried milk in 0.05% TBS-T buffer at pH 7.5 for 1 hr at room temperature. The nitrocellulose membrane was then washed, cut into strips, and separately incubated overnight at 4℃ with the patient's serum that had been diluted 1:10 with blocking solution. The membrane was then washed and incubated with goat anti-human IgE-conjugated HRP (Sigma), in the presence of blocking solution, for 1 hr at room temperature. After further washing, the membrane was incubated in SuperSignal West Pico chemiluminescent substrate (Pierce, Rockford, IL, U.S.A.) for 5 min. Fluorescence signals were detected by autoradiography using Kodak Biomax Light ML film (Eastman Kodak Company, Rochester, NY, U.S.A.).
Skin prick testing with a 1:100 dilution of Korean ginseng extract (0.01 mg/mL) induced a strong positive response to ginseng extract (histamine 4×4.5 mm, Korean ginseng extract 7×14 mm). An allergen bronchial provocation test with a 1:100 dilution of Korean ginseng extract induced no asthmatic reaction, but an early asthmatic reaction with severe coughing and dyspnea was observed 5 min after first inhaling a 1: 10 dilution of Korean ginseng extract. A physical examination revealed expiratory wheezing throughout the lung field, and FEV1 was significantly reduced versus baseline (2,430 mL vs. 3,010 mL, a 22.6% reduction) (Fig. 1).
Serum specific IgE levels to ginseng extract were significantly elevated as compared with those of 5 atopic and 5 non-atopic controls, as shown in Fig. 2 (p<0.05, O.D.=patient 1.980, atopic controls 0.110±0.170, and non-atopic controls 0.008±0.006). ELISA inhibition tests showed dose-dependent inhibition by Korean ginseng, but not by Dermatophagoides farinae, wheat flour, or Chinese balloon flower (Fig. 3).
The word Ginseng is actually used to refer to the roots of several plant species in the Araliaceae family, which include Korean ginseng (or Panax ginseng, Asian ginseng), Siberian ginseng (Eleutherococcus senticosus), and American ginseng (Panax quinquefolius) (7). Korean ginseng is the most commonly used form worldwide as an 'adaptogen'. Moreover, the ginsenosides are the main active components of ginseng and have several beneficial effects, e.g., antioxidant, anti-inflammatory, and anticancer effects (7).
A small number of cases of ginseng-induced hypersensitivity have been reported, but the IgE binding components involved have not been identified. In 1991, Brazilian ginseng was first reported to be a possible cause of respiratory hypersensitivity (4). An IgE-mediated mechanism, as confirmed by immediate skin test reactivity, a positive bronchial challenge, and by the presence of specific IgE detected by ELISA, was implicated in this case. More recently, a case of occupational asthma and rhinitis caused by Sanyak and Korean ginseng was reported (5). In this case, a 29-yr-old female herbal merchant presented with asthmatic symptoms 14 months after starting work and had also experienced angioedema of the lip, tongue, and throat after ingesting ginseng. She was diagnosed as having Sanyak and Korean ginseng-induced occupational asthma and rhinitis, which was confirmed by bronchial challenge testing. However, in this case only the Sanyak IgE binding component was detected by immunoblotting.
In the present case, ginseng-induced occupational asthma was also confirmed by bronchial challenge testing. The subject was a Korean ginseng wholesale merchant and her asthmatic symptoms were aggravated by inhaling ginseng dust, which is in line with previous cases. However, unlike previous cases, she did not develop allergic symptoms after ingesting Korean ginseng, and her duration of exposure was longer than that of the previous case (four and a half years). In the present case, skin prick testing to ginseng extract produced a strong positive response, and specific IgE to Korean ginseng was significantly elevated. Immunoblot analysis revealed four specific IgE binding components at 26, 30, 47, and 60 kDa, which were not bound by control sera. These results strongly suggest that occupational asthma induced by Korean ginseng in this subject was induced via an IgE-mediated mechanism. This is the first report to reveal the IgE binding components involved in Korean ginseng induced occupational asthma.
Figures and Tables
 | Fig. 1Allergen bronchial provocation test using a 1:10 and 1:100 dilution of Korean ginseng extract. Allergen bronchial challenge testing induced a typical immediate response. |
 | Fig. 2Measurement of serum specific IgE antibody to ginseng. The patient's serum specific IgE levels to ginseng extract were significantly higher versus those of controls. |
References
1. Park HS, Kim MJ, Moon HB. Occupational asthma caused by two herb materials Dioscorea batatas and Pinellia ternata. Clin Exp Allergy. 1994; 24:575–581.

2. Lee SK, Cho HK, Cho SH, Kim SS, Nahm DH, Park HS. Occupational asthma and rhinitis caused by multiple herbal agents in a pharmacist. Ann Allergy Asthma Immunol. 2001; 86:469–474.

3. Kim SH, Jeong H, Kim YK, Cho SH, Min KU, Kim YY. IgE-mediated occupational asthma induced by herbal medicine, Banha (Pinellia ternata). Clin Exp Allergy. 2001; 31:779–781.

4. Subiza J, Subiza JL, Escribano PM, Hinojosa M, Garcia R, Jerez M, Subiza E. Occupational asthma caused by Brazil ginseng dust. J Allergy Clin Immunol. 1991; 88:731–736.

5. Lee JY, Lee YD, Bahn JW, Park HS. A case of occupational asthma and rhinitis caused by Sanyak and Korean ginseng dusts. Allergy. 2006; 61:392–393.

6. Park HK, Jeon SG, Kim TB, Kang HR, Chang YS, Kim YK, Cho SH, Min KU, Kim YY. Occupational asthma and rhinitis induced by a herbal medicine, Wonji. J Korean Med Sci. 2005; 20:46–49.
7. Kiefer D, Pantuso T. Panax ginseng. Am Fam Physician. 2003; 68:1539–1542.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download